Which of the following statement is not correct about nitrogen?
1. Plants competes with microbes for limited nitrogen present in soil.
2. Nitrogen is a limiting nutrient for both natural and agricultural ecosystems.
3. During nitrification chemihetrotrophic bacteria play very important role.
4. Bacteria such as Pseudomonas and Thiobacillus can produce nitrogen gas from nitrate.
How many of the following statement is/are correct?
(1) Both Rhizobium and Frankia are free living in soil and can fix N2 only under symbiotic condition.
(2) Root nodules can be best observed before flowering.
(3) Formation of root nodule takes place as a result of division of pericycle cell.
(4) When Rhizobium is free living it is aerobic but when it is symbiont it becomes anaerobic to protect Mo-Fe protein.
Options:
1. 4
2. 3
3. 2
4. 1
How many of the following statement is correct about fate of ammonia in plants?
(1) At physiological pH NH3 is protonated to form NH4 which cannot be stored as it is toxic.
(2) Most plants can assimilate NH4+ as well as
(3) Assimilation of ammonia can take place by reductive amination as well as transamination.
(4) Tranport of fixed nitrogen in form of amides and ureides take place via xylem vessel.
Options :
1. 4 2. 3
3. 2 4. 1
Find odd one out with respect to Melvin Calvin
| 1. | He was professor of chemistry. |
| 2. | Calving along with J.A.Bassham studied firation of carbon in plants. |
| 3. | He proposed that plant can convert light energy into chemical energy by transferring electrons in an organized array of pigment molecules and other substance. |
| 4. | He discouraged use of radioactivity for human welfare. |
Half-leaf experiment, where a part of the leaf is enclosed in a test tube containing KOH is performed to show that:
1. H2O is required for photosynthesis
2. CO2 is required for photosynthesis
3. Light is required for photosynthesis
4. H2O is source of O2 released during photosynthesis
Match the following
|
Scientists |
Work |
||
|
(I) |
Julius van sachs |
(A) |
Only green part of plant could release oxygen. |
|
(II) |
T.W.Engelmann |
(B) |
Inferred that the O2 evolved during photosynthesis comes from H2O |
|
(III) |
Van Niel |
(C) |
First action spectrum in terms of O2 evolution. |
|
(IV) |
Ingenhousz |
(D) |
Provided evidence for production of glucose. |
Options :
| I | II | III | IV | |
| 1. | D | C | B | A |
| 2. | D | A | B | C |
| 3. | D | B | A | C |
| 4. | D | B | C | A |
Study the graph given below and find out the wrong statement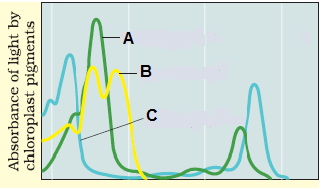
| 1. | A – chlorophyll b, B – Carotenoids, C – Chlorophyll a |
| 2 | A – Yellow-green, B – Yellow to yellow-orange, C – bright or blue-green |
| 3. | A – Major pigment, B – Accessory pigment, C – Protective pigment |
| 4. | A – Accessory pigment, B – Photoprotection, C - Major pigment |
Light reactions or the photochemical phase consists of all except :
| 1. | Absorption of light |
| 2. | Spliting of H2O and release of O2 |
| 3. | Formation of ATP and NADPH |
| 4. | Movement of Proton |
During electron transport in photosynthesis, when both PS – I and PS – II is involved, there is the formation of a characteristic Z-shape. This shape forms when?
1. All the carriers are placed in a sequence on a redox potential scale.
2. All the carriers are arranged randomly.
3. Electron from two different source gets excited simultaneously.
4. Both (1) and (3) are correct
Which of the following statement is not correct about the light reaction of photosynthesis?
1. Water splitting complex is associated with PS – II, physically located on the inner side of the membrane of the thylakoid.
2. Cyclic-photophosphorylation takes place in stroma lamellae or when the light of wavelength beyond 680 nm is the only a source of light.
3. Stroma lamellae membranes laces PS – II and NADP reductase enzyme.
4. The LHC consists of hundreds of pigment molecules bound to lipids.
During non-cyclic photophosphorylation there is transfer of electron from one complex to another. Which of the following statement is not correct?
1. Initial electron is excited from reaction centre.
2. The removed electron from reaction centre is replenished by splitting of H2O.
3. Electron removed from photosystem – I is provided by photosystem – II
4. During this process electron moves in circular pathway.
How many of the following is required for chemiosmosis?
(1) A membrane (2) A proton pump
(3) A proton gradient (4) ATPase
Options :
1. All
2. (1), (2) and (3)
3. (1), (2) and (4)
4. (1) and (2) only
Although concentration of CO2 is increased but there is no increase in rate of photosynthesis reason could be (for both C3 and C4 plants)
1. Low light intensity
2. Water stress
3. High internal CO2 concentration
4. Both (a) and (b)
The universal site of respiration in all the living organism is
1. Plasma membrane
2. Cytoplasm
3. Mitochondria
4. Chloroplast
How many of the following statement is correct for glycolysis?
(1) It is the only process of respiration in aerobic respiration.
(2) During glycolysis there is partial oxidation of glucose to form one molecule of pyruvic acid.
(3) In glycolysis, there are ten enzyme catalyzed steps.
(4) When PGAL is converted into BPGA, two redox equivalents are removed.
Options :
1. 4
2. 3
3. 2
4. 1
Which of the following statement is not correct?
1. Pyruvic acid produced as a result of glycolysis can be handled in three different ways.
2. As a result of fermentation less than 7% of energy is released, which is stored in glucose.
3. During alcoholic fermentation death of yeast takes place when alcohol concentration reaches 13%.
4. Enzyme pyruvic acid dehydrogenase and alcohol decarboxylase is involved in fermentation.
The complete oxidation of pyruvate by the stepwise removal of the all hydrogen atoms, leaving three molecules of CO2, takes place in?
1. Mitochondrial matrix
2. Inner mitochondrial membrane
3. Inter membrane space of mitochondria
4. Outer mitochondrial membrane
How many of the following statements are correct for the TCA cycle?
(1) At the first step condensation of acetyl group with OAA and H2O takes place.
(2) Isocitrate undergoes two successive decarboxylations leading to the formation of a-ketoglutaric acid and then succinyl – CoA
(3) Substrate level phosphorylation takes place when succinyl CoA gets converted into fumaric acid.
(4) In a single turn TCA produces 6-NADH, 2-FADH, and 2-GTP.
Options :
1. 4
2. 3
3. 2
4. 1
Find out odd statement about electron transport system.
| 1. | It is located in inner mitochondrial membrane and consists of four different complexes. |
| 2. | During this process two mobile carrier that areUQ and Cytc. |
| 3. | Cyt a – a3 contains two copper centeres and acts as terminal acceptor of electron. |
| 4. | O2 acts as a final hydrogen acceptor. |
What could be the possible reasons for the production of less than 36-ATP during aerobic respiration of glucose?
1. pathway was not sequential
2. Cytoplasmic NADH was not transferred to mitochondria
3. Some of the intermediates of the pathway was utilized
4. Respiratory substrate was other than glucose
Options :
1. 1, 2, 3 and 4
2. 1, 2 and 3
3. 2, 3 and 4
4. 1, 3 and 4
After degradation by protease proteins enter into aerobic pathway at which of the following stage?
1. Some stage within Kreb cycle
2. As pyruvate
3. As acetyl CoA
4. All the above
Which of the following statement is incorrect?
1. RQ value provided information about nature of respiratory substrate.
2. RQ value of carbohydrate = 1, Fat = 0.7 and that of protein = 0.9.
3. In living organism respiratory substrate is often more than one.
4. Generally pure protein and pure fat is used as a respiratory substrate
The form of growth in which new cells are continuously added is known as
1. Diffuse form of growth
2. Open form of growth
3. Continuous form of growth
4. Ring form of growth
Which of the following is not the characteristics of cells present in the meristematic phase
1. Cells densely protoplasmic with a large nucleus
2. Cell wall thin and cellulosic
3. the Increased amount of vacuolation
4. Presence of abundant plasmodesmata connections
The exponential growth can be expressed as w1 = w0ert, where r is?
1. Ability of plant to produce new plant material
2. Efficiency index
3. Growth rate
4. All of these
Find incorrect match
| 1. | Differentiation – specialization |
| 2. | De differentiation – Regain capacity to divide again |
| 3. | Rediffrentiation – Specialisation of dedifferentiated cell |
| 4. | Relative growth – Final size |
Match the following
PGR Derivative
(A) Auxin (1) Terpenes
(B) Cytokinin (2) Carotenoides
(C) ABA (3) Purine
(D) GA3 (4) Amono acid
Options :
A B C D
1. 1 2 3 4
2. 4 2 3 1
3. 4 3 2 1
4. 1 4 3 2
Following are the statements related to PGR how many of them are correct?
(1) Auxin is responsible for phototropism.
(2) Sterile filtrates of the fungus Gibberella fujikuroi can break seed dormancy.
(3) A ripened orange can cause sprouting in potato.
(4) Extract of maize endosperm can overcome apical dominance.
1. 4
2. 3
3. 2
4. 1
Which of the following hormone promotes flowering in pineapple
1. IAA
2. C2H2
3. GA3
4. Both (1) and (2)
During flowering shoot apices modify themselves to flowering apices. The photoperiod is perceived by
1. Shoot apices
2. Mature leaves
3. Stem
4. Stomata
Which of the following statement is incorrect?
1. Diffusion is a slow process and not dependent upon a living system.
2. Diffusion across the membrane depends upon its solubility in lipids.
3. Porins are proteins that forms huge pores, in the inner membrane of the plastids, mitochondria and some bacteria.
4. Water channels are made up of eight different aquaporins.
Following are two statements, find the correct option
(I) In a flowering plant there is complex traffic of compounds moving in different directions.
(II) Each organ of a plant receives some substance and gives out some
Option :
1. Both (I) and (II) are correct
2. Only (I) is correct
3. Only (II) is correct
4. Neither (I) nor (II) is correct
Which of the following is not correct for imbibitions?
1. Imbibition plays a very important role in the emergence of the seedling.
2. In imbibition movement of the solvent is against the gradient.
3. Water potential gradient is essential for imbibition.
4. Affinity between liquid and solid is a pre-requisite for imbibitions.
How many of the following statements are correct?
(1) Vacuolar sap contributes to the solute potential of cells.
(2) The net direction and rate of osmosis depend upon the pressure gradient and concentration gradient.
(3) Osmotic pressure is a function of the solute concentration.
(4) Osmotic pressure is a positive pressure while the osmotic potential is negative.
Options :
1. 4
2. 3
3. 2
4. 1
How many of the following statements are correct?
(1) Less than one percent of water reaching the leaves is used in photosynthesis and plant growth.
(2) Turgidity of guard cells plays a very important role in stomatal opening and closing.
(3) In the dorsiventral leaf upper surface has a greater number of stomata.
(4) In guard cells cellulosic microfibrils are oriented radially.
Option :
1. 4
2. 3
3. 2
4. 1
The C4 plants are twice efficient with respect to C3 plants because
| 1. | They have ability to maximize availability of CO2 while minimizing H2O loss. |
| 2. | They have low CO2 requirement with minimum H2O loss. |
| 3. | There stomata remains always open and high water loss. |
| 4. | There stomata open during night, and minimum H2O loss. |
Match the following :
|
(1) |
Sink of nutrient |
(A) |
Fine vein endings |
|
(2) |
Unloading of mineral |
(B) |
Lateral meristeny |
|
(3) |
Mineral remobilisation |
(C) |
Storage organs |
|
(4) |
Unloading of sugar |
(D) |
Older parts |
Options :
1 2 3 4
1. A B C D
2. B A D C
3. A C B D
4. B C A D
Older dying leaves export much of their mineral content to younger leaves. Similarly, what is incorrect-?
1. Before leaf fall in deciduous plants, minerals are removed to other parts.
2. Elements most readily mobilized are phosphorus, nitrogen, and potassium.
3. Some elements that are structural components like calcium are not remobilized.
4. Deficiency symptoms of nitrogen are observed in younger parts.
Which of the following statement is not correct for toxicity of mineral nutrients :
1. Toxicity is mainly associated with micro nutrients.
2. Toxicity level of any nutrient also varies for different plants.
3. Toxicity of one nutrient may inhibit uptake of another nutrient.
4. Symptom of toxicity cannot be due to deficiency of any other nutrient.
Zinc is essential for
1. Biosynthesis of chlorophyll
2. Biosynthesis of auxin
3. Stomatal closing
4. Oxidation of carbohydrates
Blackman demonstrated that increasing illumination increased the photosynthetic rate up to a point when CO2 becomes limiting. If the light was not limiting, temperature becomes limiting. Emerson found that maximum CO2 fixation could be achieved with brief flashes of light. Mark the correct statement in the following
| 1. | Only one factor can be limited in photosynthesis |
| 2. | Photosynthesis consists of a light and dark reaction |
| 3. | The trapping of light by chloroplast is temperature-dependent |
| 4. | The trapping of light by chloroplast can occur only if CO2 is present |
The thylakoids are removed and kept in a culture medium containing CO2 and H2O. If the setup is exposed to light, hexose sugars are not formed as the end product. The most appropriate reason for this will be that
| 1. | Carbon assimilation cannot take place |
| 2. | The pigments (P–700 and P–680) are not linked |
| 3. | Enzymes are not available |
| 4. | The light-trapping device is not functional |
How many of the following statement is correct about respiration?
I. Respiratory substrate is often more than one in a living organisms.
II. Pure protein or pure fat can never be respiratory substrates.
III. Protein has a maximum possible option for entry into the respiratory pathway.
IV. Acetyl CoA can be a product or precursor of fatty acid
Option :
1. 4
2. 3
3. 2
4. 1
During electron transport in mitochondria, which statement is not correct about complex – IV
1. It is the terminal donor of an electron to oxygen.
2. It is also known as cytochrome oxidase
3. It contains Cu, which is reversibly oxidised from
4. It receives an electron from UQ.
Substrate level phosphorylation during TCA cycle occurs :
1. Once
2. Twice
3. 3 times
4. 6 times
A second messenger will not be involved in the action of which of the following hormones on its target cells?
1. Adrenocorticotropin
2. Adrenaline
3. Thyroxin
4. Thyrocalcitonin
The releasing and inhibiting hormones synthesized by hypothalamus are transported from the hypothalamus to the anterior pituitary by way of:
1. the general bloodstream
2. a portal system of blood vessels
3. axons that are present in the pituitary stalk or infundibulum
4. transport carrier proteins present in the CSF
Which of the following correctly describes a negative feedback system?
1. As hormone levels rise, hormone release is promoted.
2. Target organ effects inhibit further hormone release.
3. As hormone levels decrease, hormone release is promoted.
4. As hormone levels decrease, hormone release is inhibited.
All the following are a type of stimulus to trigger endocrine glands to manufacture and release their hormones except:
1. hormonal stimuli
2. neural stimuli
3. permissive stimuli
4. humoral stimuli
A deficiency of both glucocorticoids and mineralocorticoids will cause:
1. Cushing's syndrome
2. Conn’s syndrome
3. Graves' disease
4. Addison's disease
Which of the following is not a metabolic function of human Growth Hormone?
1. Promotes use of blood glucose by body cells
2. Promotes protein anabolism
3. Promotes lipolysis
4. Promotes gluconeogenesis in liver
In spite of a high level of prolactin during pregnancy, milk production does not start because:
1. A high prolactin level inhibits the secretion of GnRH
2. High levels of progesterone are present during pregnancy
3. High hCG levels do not allow mammary alveoli to function
4. The receptors for prolactin are downgraded during pregnancy
Which of the following hormones will be antagonistic to insulin in its effects on carbohydrate, fat and protein metabolism?
1. Cortisol
2. Glucagon
3. Human Growth Hormone
4. Thyroxin
A medical condition that is most likely to be associated with a relative deficiency of vitamins would be:
1. Hyperthyroidism
2. Acromegaly
3. Cretinism
4. Diabetes mellitus
You would expect an enlarged and abnormal thymus gland in patients of:
1. HIV
2. SCID
3. Cushing’s disease
4. Myasthenia gravis
A potentially life threatening respiratory failure can occur in cases of severe:
1. hyperparathyroidism
2. hyperthyroidism
3. hypothyroidism
4. hypoparathyroidism
Which of the following hormones may be used in cases of sleep disturbances such as due to a jet-lag?
1. Adrenaline
2. Thymosin
3. Melatonin
4. Thyroxin
Hyperpigmentation of skin is seen in:
1. Myeloma
2. Addisson’s disease
3. Hashimoto’s disease
4. Pituitary dwarfism
A young patient is suffering from high blood pressure and muscle weakness. Which region of the adrenal gland directly or indirectly is causing these signs and symptoms?
1. hypersecretion by the zona glomerulosa
2. hypersecretion by the zona fasciculata
3. hypersecretion by the zona reticularis
4. hyposecretion by the zona glomerulosa
The production of thyrotropin-releasing hormone can be promoted by:
1. increased levels of ATP production in the body
2. lower-than-normal body temperatures
3. increased levels of T3 and T4 in blood
4. an increase in appetite with weight gain
An increased secretion of growth hormone say due to a tumor in anterior pituitary would cause all the following except:
1. acromegally in adults
2. giantism in children
3. diabetes
4. suppression of cancer
A hormone produced by the skin in human beings would be:
1. melanin
2. cholecalciferol
3. melatonin
4. erythropoietin
Transport of glucose into the cells depends on the presence of insulin in all of the following tissues except:
1. skeletal muscle
2. adipose tissue
3. the brain
4. the myocardium
Osteitis fibrosa cystica is caused by:
1. an increase in ADH
2. an increase in the parathyroid hormone
3. an increase in the growth hormone in an adult
4. an increase in calcitonin
An action potential for a given axon is:
1. different in size each time it occurs
2. always the same size
3. larger when the information is to be carried faster
4. smaller when information goes to a gland rather than a muscle
The axons of invertebrate animals do not have myelin sheaths. This is compensated by having:
1. very long axons
2. very short axons
3. axons of very large diameter
4. axons of very small diameter
Alzheimer's disease is characterized by all the following except:
1. toxic beta amyloid peptide build up in brain
2. shrinkage of all the brain tissue
3. increased levels of acetylcholine in the basal forebrain
4. tau protein abnormalities
Sorting and editing of sensory impulses to cerebrum takes place in:
1. pons
2. cerebellum
3. thalamic nuclei
4. hypothalamus
Pons:
1. acts to regulate body temperature
2. provides motor signals to the cerebrum
3. controls vomiting and coughing
4. contains nuclei that relay information from the cerebrum to cerebellum
Area of the brain, most involved in maintaining the body's homeostasis, is:
1. medulla oblongata
2. cerebellum
3. pons
4. hypothalamus
If you visually follow a moving object, the part of the brain that coordinates head and eye movements will be:
1. occipital cortex
2. superior colliculi
3. cerebellum
4. thalamus
Cerebral aqueduct passes through the:
1. Mid brain
2. Diencephalon
3. Hind brain
4. Spinal cord
Sympathetic stimulation does not lead to:
1. increased respiratory rate
2. activation of energy reserves
3. increased metabolic rate
4. increased storage of lipid and glycogen
Bipolar neurons are found in:
1. Olfactory membrane
2. Choroid of eye
3. Dorsal root ganglion of spinal nerves
4. Cerebellar peduncles
Parasympathetic stimulation does not result in:
1. sexual arousal and stimulation of sexual glands
2. constriction of respiratory passageways
3. dilation of the pupils
4. an increase in smooth muscle activity along the digestive tract
If there is no stimulus, the autonomic motor neurons:
1. show a background level of activity
2. are completely active
3. are completely inhibited
4. are completely inhibited and are completely active
The eye ball has three layers. What would respectively be the feature and name of the middle layer?
1. secretory; choroid
2. vascular; uvea
3. nervous; retina
4. fibrous; sclera
Normal accommodation function of the human eye is called as:
1. emmetropia
2. presbyopia
3. hyperopia
4. myopia
In retina of human eye, a nerve impulse travels from the:
1. ganglionic cells to bipolar cells to rods and cones
2. rods and cones to bipolar cells to ganglionic cells
3. bipolar cells to rods and cones to ganglionic cells
4. rods and cones to ganglionic cells to bipolar cells
The organ of Corti is found in the:
1. cochlear duct
2. middle ear
3. semicircular canal
4. utricle
The Nissl’s granules are found in:
I. Dendrites
II. Soma or cell body
III. Axon
1. Only I
2. Only II
3. I and II only
4. I, II and III
Unmyelinated neurons:
1. are not surrounded by Schawann cells and myelin sheath
2. are surrounded by Schwann cells that do not secrete myelin sheath
3. are surrounded by oligodendrocytes rather than Schwann cells
4. are adapted for faster conduction of impulses than the myelinated neurons
A motor neuron and all the muscle fibers it supplies is called:
1. motor unit
2. neuromuscular junction
3. motor end plate
4. fascicle
After its release at the neuromuscular junction, Acetylcholine binds to the motor end plate causing a change in membrane permeability to:
1. potassium
2. calcium
3. sodium
4. magnesium
A feature unique to smooth muscles is the presence of:
1. T tubules
2. multiple nuclei
3. Calmodulin
4. myosin and actin filaments
If production of pyruvic acid by anaerobic metabolism in a muscle is faster than it can be utilized, the surplus is converted to:
1. lactic acid
2. adenosine diphosphate
3. carboxylic acid
4. creatine phosphokinase
Immunosupression can be a treatment option in cases of:
1. Muscular dystrophy
2. Myasthenia gravis
3. Osteoporosis
4. Gouty arthritis
Identify the incorrectly matched pair:
| Joint type | Example | |
| 1. | Hinge | Knee |
| 2. | Pivot | Between atlas and axis vertebra |
| 3. | Gliding | Between the metacarpals |
| 4. | Saddle | Between carpal and metacarpal of thumb |
The number of which of the following would normally be equal in human skeleton?
1. Vertebrochondral ribs and digits in both upper limbs
2. Bones in cranium and the facial skeleton
3. Carpals and tarsals
4. Vertebrosternal ribs and digits in one lower limb
The human ribs are termed as ‘bicephalic’ because:
1. They attach to other bones both dorsally and ventrally
2. They have two articulation surfaces on their dorsal end
3. They have two articulation surfaces on their ventral end
4. They articulate with the help of cartilage at both ends
The Hf0 for CO2 (g), CO(g) and H2O(g) are -393.5, -110.5 and -241.8 kJ mol–1 respectively. The standard enthalpy change (in kJ) for the reaction
CO2 (g) + H2 (g) CO(g) + H2O(g) is -
1. 524.1
2. 41.2
3. -262.5
4. -41.2
In thermodynamics, a process is called reversible when -
1. surroundings and system change into each other
2. there is no boundary between system and surroundings
3. the surroundings are always in equilibrium with the system
4. the system changes into the surroundings spontaneously
One mole of a non-ideal gas undergoes a change of state (2.0 atm, 3.0L, 95K) (4.0 atm, 5.0L, 245 K) with a change in internal energy,
E = 30.0 L atm. The change in enthalpy (H) of the process in L atm is -
1. 40.0
2. 42.3
3. 44.0
4. not defined, because pressure is not constant
Which of the following equation gives the value of heat of formation (Hºf)
1. H2(g) + F2 (g) 2HF(g)
2. H2 (g) + F2 (g) HF(g)
3. H + F HF
4. Information is not sufficient
For a reversible process at T = 300 K, the volume is increased from V = 1L to Vf = 10L. Calculate H if the process is isothermal.
1. 11.47 KJ
2. 4.98 KJ
3. 0
4. –11.47 KJ
For the reaction
X2O4 () 2XO2 (g) E = 2.1 kcal,
S = 20 cal/K at 300 K. Hence G is–
1. 2.7 kcal
2. – 2.7 kcal
3. 9.3 kcal
4. – 9.3 kcal
5 mol of ideal gas at 27ºC expands isothermally and reversibly from a volume of 61 to 601. The work done in kJ is–
1. – 14.7
2. – 28.72
3. + 28.72
4. – 56.72
10 mole of ideal gas confined to a volume of 10 L is released into atmosphere at 300 K where the pressure is 1 bar. The work done by the gas is (R = 0.083 L- bar k–1 mol–1).
1. 249 L-bar
2. 259 L-bar
3. 239 L-bar
4. 220 L-bar
Enthalpy of formation of 2 mol of NH3 (g) is
– 90 kJ and HH–H, HN–H are respectively 435 kJ mol–1 and 390 kJ mol–1. The value of H is–
1. – 472.5 kJ
2. – 945 kJ mol–1
3. 472.5 kJ
4. 945 kJ mol–1
Difference between H and U for the combustion of benzene at 300 K is –
1. 7.48 kJ
2. 3.74 kJ
3. 14.86 kJ
4. 5.73 kJ
If S + O2 SO2,
H = – 298.2 kJ mole–1
SO2 + 1/2 O2 SO3,
H = – 98.7kJmole–1
SO3 + H2O H2SO4 ,
H = – 130.2 kJ mole–1
H2 + 1/2 O2 H2O,
H = – 287.3 kJ mole–1
the enthalpy of formation of H2SO4 at 298 K will be–
1. – 814.4 kJ mole–1
2. + 814.4 kJ mole–1
3. – 650.3 kJ mole–1
4. – 433.7 kJ mole–1
2.1 g of Fe combines with S evolving 3.77 kJ. The heat of formation of FeS in kJ/mole is–
1. – 3.77
2. – 1.79
3. – 100.5
4. none of these
For the reversible reaction,
N(g) +3H(g) 2NH(g) at 500ºC,
the value of K is 1.44 × 10 when partial pressure is measured in atmospheres. The corresponding value of K, with concentration value of K, with concentration in
mole litre, is-
1. 1.44 × 10 / (0.082 × 500)
2. 1.44 × 10 / (8.314 × 773)
3. 1.44 × 10 / (0.082 × 773)
4. 1.44 × 10 / (0.082 × 773)
At constant temperature the equilibrium constant K for the decomposition reaction NO 2NO is expressed by K = where P = pressure, x = extent of decomposition which of the following statements is true ?
1. increases with increase of P
2. K increases with increases of x
3. K increases with decrease of x
4. K remains constant with change in P&X
At a certain temperature, equilibrium constant (K) is 16 for the reaction ;
SO(g) + NO(g) SO(g) + NO(g)
If we take one mole of each of all the four gases in a one litre container, what would be the equilibrium concentrations of NO and NO?
1. 1.6 mol/L
2. 2.3 mol/L
3. 4.8 mol/L
4. None of these
In a vessel containing SO, SO and O at equilibrium, some helium gas is introduced so that the total pressure increases while temperature and volume remains constant. According to Le-chatelier principle, the dissociation of SO -
1. increase
2. decrease
3. remain unaltered
4. changes unpredictable
Given a system in equilibrium, an increase in concentration of the product is always produced by a rise in temperature when the reaction is -
1. Gas phase
2. Spontaneous
3. Endothermic
4. First order
Nitrogen gas was injected into an equilibrium mixture of
2SO(g) 2SO(g) + O(g)? The pressure is increased from 1.0 atm to 10 atm. Which of the following statements is correct -
1. The concentration of the reacting gases are unchanged.
2. [SO] increases
3. [SO] increases
4. [O] increases
According to Le-Chatelier’s principle, adding heat to a solid to liquid in equilibrium will causes the -
1. Temperature to increase
2. Temperature to decrease
3. Amount of liquid to increase
4. Amount of solid to increase
N2O4 is 25% dissociated at 37ºC and one atmosphere pressure. Calculate (i) Kp and (ii) the percentage dissociation at 0.1 atmosphere and 37ºC.
1.
2.
3.
4. None of above
A sample of air consisting of and was heated to 2500 K until the equilibrium
was established with an equilibrium constant At equilibrium, the mole % of NO was 1.8. Estimate the initial composition of air in mole fraction of and .
1. N2 = 79%, O2 =21%
2.N2 = 69%, O2 =31%
3. N2 = 55%, O2 =45%
4. N2 = 79%, O2 =17%
The progress of the reaction
A nB with time, is presented in figure. Determine the value of n.
1. 2
2. 3
3. 4
4. None of these
For the reaction: N2O4(g)2NO2(g),
H = 14.6 kcal. If Kp = 0.141 at 298 K, calculate Kp at 338 K.
1. 2.1
2. 3.5
3. 4.6
4. None of the above
Solid Ammonium carbamate dissociates as : NH2COONH4(s) 2NH3(g) + CO2(g). In a closed vessel solid ammonium carbamate is in equilibrium with its dissociation products. At equilibrium, ammonia is added such that the partial pressure of NH3 at new equilibrium now equals the original total pressure. Calculate the ratio of total pressure at new equilibrium to that of original total pressure.
1.
2.
3.
4. None of the these
A sample of CaCO3(s) is introduced into a sealed container of volume 0.821 litre and heated to 1000 K until equilibrium is reached. The equilibrium constant for the reaction CaCO3(s) CaO(s) + CO2(g) is 4 × 10–2 atm at this temperature. Calculate the mass of CaO present at equilibrium.
1. 25 mg
2. 23.2 mg
3. 22.4 mg
4. None of these
The number of moles of oxalate ions oxidized by one mole of MnO4– ion is -
1. 1/5 2. 2/5
3. 5/2 4. 5
What weight of nitrate ion (calculated as HNO3) is needed to convert 5g of iodine into iodic acid according to the reaction -
I2 + HNO3 HIO3 + NO2 + H2O
1. 12.4 g
2. 24.8 g
3. 0.248 g
4. 49.6 g
What volume of 3 molar HNO3 is needed to oxidise 8 g of Fe2+ to Fe3+ ?
HNO3 NO
1. 8 ml
2. 15.87 ml
3. 32 ml
4. 64 ml
How many litres of Cl2 at S.T.P. will be liberated by the oxidation of NaCl with 10 g KMnO4 ?
1. 3.54 litres
2. 7.08 litres
3. 1.77 litres
4. None of these
When the ion acts as an oxidant in acidic aqueous solution the ion Cr is formed. How many moles of Sn would be oxidised to by one mole of ions -
1. 2/3
2. 3/2
3. 2
4. 3
What is the equivalent weight of P in the following reaction ?
P + NaOH NaH PO + PH
1000 g aqueous solution of CaCO3 contains
10 g of calcium carbonate. Hardness of the solution is -
1. 10 ppm
2. 10 ppm
3. 1000 ppm
4. 10000 ppm
When the same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volumes of hydrogen evolved is -
1. 1 : 1
2. 1 : 2
3. 2 : 1
4. 9 : 4
The volume of '10 Vol' of H2O2 required to liberate 500 mL O2 at NTP is -
1. 50 ml
2. 25 ml
3. 100 ml
4. 125 ml
When 50% solution of H2SO4 is electrolysed by passing a current of high density at low temperature the main products of electrolysis are -
1. Oxygen and hydrogen
2. H2 and peroxy disulphuric acid
3. H2 and SO2
4. O2 and peroxy disulphuric acid
MgSO4 + NH4Cl + Na2HPO4 White crystalline precipitate.
1. MgCl2.MgSO4
2. MgSO4
3. Mg(NH4)PO4
4. Mg(PO4)2
Halides of alkaline earth metals form hydrates such as MgCl2.6H2O, CaCl2.6H2O, BaCl2.2H2O and SrCl2.2H2O. This shows that halides of group 2 elements -
1. are hygroscopic in nature
2. can act as dehydrating agents
3. can absorbs moisture from air
4. all of the above
A metal X on heating in nitrogen gas gives Y. Y on treatment with H2O gives a colourless gas which when passed through CuSO4 solution gives a blue colour. Y is -
1. Mg(NO3)2
2. Mg3N2
3. NaN3
4. MgO
Nitrogen dioxide cannot be prepared by heating
1. KNO3
2. Pb(NO3)2
3. Cu(NO3)2
4. AgNO3
Solution of sodium metal in liquid ammonia is strongly reducing due to the presence of the following in solution -
1. Sodium hydride
2. Sodium amide
3. Sodium atoms
4. Solvated electrons
Which of the following statements about H2O2 is not true -
1. H2O2 is used to clean oil paintings
2. H2O2 acts as oxidising as well as reducing agent
3. Two hydroxyl groups in H2O2 lie in the same plane
4. It retains same structure in liquid and solid form
When CO is heated with NaOH under pressure, we get -
1. Sodium benzoate
2. Sodium acetate
3. Sodium formate
4. Sodium oxalate
PbCl4 exists but PbBr4 and PbI4 do not because of -
1. Inability of bromine and iodine to oxidise Pb2+ to Pb4+
2. Br– and I– are bigger in size
3. More electropositive character of Br2 and I2
4. Chlorine is a gas
In silicon dioxide -
1. Each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bonded to two silicon atoms
2. Each silicon atom is surrounded by two oxygen atoms and each oxygen atom is bonded to two silicon atoms
3. Silicon atom is bonded to two oxygen atoms
4. There are double bonds between silicon and oxygen atoms
Silica reacts with Mg to form a magnesium compound (A). (A) react with dil. HCl and forms (B). (B) is -
1. MgSiO 2. MgO
3. MgCl2 4. SiCl4
Two particles of equal masses go around a circle of radius \(R\) under the action of their mutual gravitational attraction. The speed of each particle is:
1. \(v = \frac{1}{2 R} \sqrt{\frac{1}{Gm}}\)
2. \(v = \sqrt{\frac{Gm}{2 R}}\)
3. \(v = \frac{1}{2} \sqrt{\frac{G m}{R}}\)
4. \(v = \sqrt{\frac{4 Gm}{R}}\)
The distance of the centres of moon and earth is D. The mass of earth is 81 times the mass of the moon. At what distance from the centre of the earth, the gravitational force will be zero
1.
2.
3.
4.
Mass \(M\) is divided into two parts \(xM\) and \((1-x)M.\) For a given separation, the value of \(x\) for which the gravitational attraction between the two pieces becomes maximum is:
| 1. | \(\frac{1}{2}\) | 2. | \(\frac{3}{5}\) |
| 3. | \(1\) | 4. | \(2\) |
Two identical solid copper spheres of radius R placed in contact with each other. The gravitational attracton between them is proportional to
1. R2
2. R–2
3. R4
4. R–4
| 1. | \(32\) N | 2. | \(56\) N |
| 3. | \(72\) N | 4. | zero |
The escape velocity of a particle of mass \(m\) varies as:
| 1. | \(m^{2}\) | 2. | \(m\) |
| 3. | \(m^{0}\) | 4. | \(m^{-1}\) |
A \(4.0\) m long copper rod of cross-sectional area \(1\) cm2 is stretched by a force of \(4.8\times 10^3~\mathrm{N}\). If Young’s modulus for copper is \(Y=1.2\times 10^{11}~ \mathrm{N/m^2}\). The strain will be:
1. \(1\times 10^{-4}\)
2. \(2\times 10^{-4}\)
3. \(3\times 10^{-4}\)
4. \(4\times 10^{-4}\)
When a certain weight is suspended from a long uniform wire, its length increases by one cm. If the same weight is suspended from another wire of the same material and length but having a diameter half of the first one, the increase in length will be:
1. 0.5 cm
2. 2 cm
3. 4 cm
4. 8 cm
A force F is needed to break a copper wire having radius R. The force needed to break a copper wire of the same length and radius 2R will be:
1. F/2
2. 2F
3. 4F
4. F/4
Two rods of different materials having coefficients of linear expansion 1 and 2 and Young’s modulli, Y1 and Y2 respectively are fixed between two rigid massive walls. The rods are heated such that they undergo the same increase in temperature. There is no bending of rods. If , then the thermal stresses developed in the two rods are equal, provided is equal to
1. 2 : 3 2. 1 : 1
3. 3 : 2 4. 4 : 9
Two wires of the same material and length are stretched by the same force. Their masses are in the ratio 3 : 2, Their elongations are in the ratio
1. 3 : 2
2. 9 : 4
3. 2 : 3
4. 4 : 9
A 1000 kg lift is tied with metallic wires of maximum safe stress of 1.4 108 N m-2. If the maximum acceleration of the lift is 1.2 m s-2, then the minimum diameter of the wire is:
1. 1 m
2. 0.1 m
3. 0.01 m
4. 0.001 m
The pressure of water in a water pipe when tap is opened and closed are respectively \(3\times10^5\) Nm–2 and \(3.5\times10^5\) Nm–2. With open tap, the velocity of water flowing is:
1. \(10\) ms–1
2. \(5\) ms–1
3. \(20\) ms–1
4. \(15\) ms–1
Two soap bubbles of radii R and r come in contact. R is more than r. Radius of curvature of common surface is
1.
2.
3.
4.
A cubical block of wood of the side \(10\) cm floats at the interface between oil and water with its lower surface horizontal and \(4\) cm below the interface. The density of oil is \(0.6\) g cm–3. The mass of block is:
1. \(706\) g
2. \(607\) g
3. \(760\) g
4. \(670\) g
A mercury-filled U–tube arrangement is connected to a bulb containing a gas. Atmospheric pressure is \(1.012 \times 10^5\) Pa and \(\mathrm{H}=0.05\) m.
Then:
| 1. | gauge pressure at \(\mathrm{R}\) is nil. |
| 2. | gauge pressure at \(\mathrm{R}\) is \(6.56 \times10^3\) Pa. |
| 3. | gauge pressure at \(\mathrm{R}\) is \(1.08 \times10^5\) Pa. |
| 4. | pressure at \(\mathrm{R},\) \(\mathrm{Q},\) and inside the bulb is the same. |
Density of sea water is 1.03 × 103 kg m–3. A ship of weight 10.1 × 106 N floats on it. The ship then enters the fresh water and some cargo is unloaded so that volume of ship submerged in sea water and fresh water is same, then-
1. volume of sea water displaced is 103 m3
2. mass of sea water displaced is 3 × 104 kg
3. A is incorrect
4. B is correct.
A wire can sustain a weight of 10 kg before breaking. If the wire is cut into two equal parts, then each part can sustain a weight of:
| 1. | 2.5 kg | 2. | 5 kg |
| 3. | 10 kg | 4. | 15 kg |
A body weighs 160 g in air, 130 g in water and 136 g in oil. The specific gravity of oil is
1. 0.2
2. 0.6
3. 0.7
4. 0.8
A diver is \(10\) m below the surface of the water. The approximate pressure experienced by the diver is:
1. \(10^5\) Pa
2. \(2\times10^5\) Pa
3. \(3\times10^5\) Pa
4. \(4\times 10^5\) Pa
A penguin floats first in a fluid of density 0, then in a fluid of density 0.950 and then in a fluid of density 1.10. Which of the following is correct ?
1. maximum buoyant force in the fluid of density of 0.950
2. maximum buoyant force in the case of fluid of density 1.10
3. maximum fluid is displaced in the case of density 1.1r0
4. maximum fluid is displaced in the case of density 0.950
A piece of brass (Cu and Zn) weighs 12.9 g in air. When completely immersed in water, it weighs 11.3 g. The relative densities of Cu and Zn are 8.9 and 7.1 respectively. The mass of copper in the alloy is
1. 4.6 g
2. 5.6 g
3. 7.6 g
4. 8.6 g
Two soap bubbles, each with a radius r, coalesce in vacuum under isothermal conditions to form a bigger bubble of radius R. Then R is equal to
1. 2-1/2 r 2. 21/3 r
3. 21/2 r 4. 2 r
Three rods made of the same material and having the same cross-section have been joined as shown . Each rod is of the same length. The left and right ends are kept at 0C and 90C respectively. The temperature of the junction of the three rods
1. 45C
2. 60C
3. 30C
4. 20C
A substance is in solid form at \(0^{\circ}\mathrm{C}\). The amount of heat added to this substance and its temperature are plotted in the following graph. If the relative specific heat capacity of the solid substance is 0.5, from the graph, the specific latent heat of the melting process is: (Specific heat capacity of water = 1000 cal kg-1 K-1 )
| 1. | 60000 cal kg-1 | 2. | 40000 cal kg-1 |
| 3. | 10000 cal kg-1 | 4. | 20000 cal kg-1 |
A substance is in the solid form at \(0^{\circ}\text{C}\). The amount of heat added to this substance and its temperature are plotted in the following graph. If the relative specific heat capacity of the solid substance is \(0.5\), find from the graph, the specific heat of the substance in the liquid state is
(Specific heat capacity of water = \(1000~\text{cal kg}^{-1}\text{K}^{-1}\))
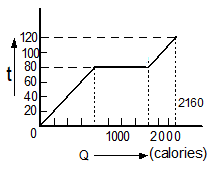
1. \(300~\text{cal kg}^{-1}\text{K}^{-1}\)
2. \(500~\text{cal kg}^{-1}\text{K}^{-1}\)
3. \(700~\text{cal kg}^{-1}\text{K}^{-1}\)
4. \(100~\text{cal kg}^{-1}\text{K}^{-1}\)
Some water at \(0^{\circ}\mathrm{C}\) is placed in a large insulated enclosure (vessel). The water vapour formed is pumped out continuously. What fraction of the water will eventually freeze, if the latent heat of vaporization is seven times the latent heat of fusion?
| 1. | 7/8 | 2. | 8/7 |
| 3. | 3/8 | 4. | 5/8 |
Find the coefficient of volume expansion for an ideal gas at constant pressure.
1.
2.
3.
4.
During an experiment, an ideal gas is found to obey an additional law VP2 = constant. The gas is initially at temperature T and volume V. What will be the temperature of the gas when it expands to a volume 2V?
1.
2.
3.
4.
We have two vessels of equal volume, one filled with hydrogen and the other with equal mass of helium. The common temperature is \(27^{\circ}\text{C}.\) What is the relative number of molecules in the two vessels?
1. \(\frac{n_\mathrm{H}}{n_\mathrm{He}} = \frac{1}{1}\)
2. \(\frac{n_\mathrm{H}}{n_\mathrm{He}} = \frac{5}{1}\)
3. \(\frac{n_\mathrm{H}}{n_\mathrm{He}} = \frac{2}{1}\)
4. \(\frac{n_\mathrm{H}}{n_\mathrm{He}} = \frac{3}{1}\)
We have two vessels of equal volume, one filled with hydrogen and the other with equal mass of Helium. The common temperature is 27oC.
If pressure of Hydrogen is 2 atm, what is the pressure of Helium ?
1.
2.
3.
4.
We have two vessels of equal volume, one filled with hydrogen and the other with equal mass of Helium. Initially the common temperature is 27oC. If the temperature of Helium is kept at 27 C and that of hydrogen is changed, at what temperature will its pressure become equal to that of helium ? The molecular weights of hydrogen and helium are 2 and 4 respectively.
1. -123C
2. -140C
3. -160C
4. -183C
The pressure of a monoatomic gas increases linearly from \(4\times 10^5~\text{N/m}^2\) to \(8\times 10^5~\text{N/m}^2\) when its volume increases from \(0.2 ~\text m^3\) to \(0.5 ~\text m^3.\) The work done by the gas is:
1. \(2 . 8 \times10^{5}~\text J\)
2. \(1 . 8 \times10^{6}~\text J\)
3. \(1 . 8 \times10^{5}~\text J\)
4. \(1 . 8 \times10^{2}~\text J\)
The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. Calculate increase in internal energy of gas.
1.
2.
3.
4.
The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. The amount of heat supplied is:
1.
2.
3.
4.
The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. Calculate molar heat capacity of the gas [R = 8.31 J/mol k]
1. 20.1 J/molK
2. 17.14 J/molK
3. 18.14 J/molK
20.14 J/molK
Two cylinders \(A\) and \(B\) fitted with pistons contain equal amounts of an ideal diatomic gas at \(300~\text K.\) The piston \(A\) is free to move, while that of \(B\) is held fixed. The same amount of heat is given to the gas in each cylinder. If the rise in temperature of the gas in \(A\) is \(30~\text K,\) then the rise in temperature of the gas in \(B\) is:
| 1. | \(30~\text K\) | 2. | \(18~\text K\) |
| 3. | \(50~\text K\) | 4. | \(42~\text K\) |
80 gm of water at 30C is poured on a large block of ice at 0C. The mass of ice that melts is
1. 30 gm
2. 80 gm
3. 150 gm
4. 1600 gm
The volume \(V\) versus temperature \(T\) graph for a certain amount of a perfect gas at two pressures \(P_1\) and
\(P_2\) are shown in the figure.

Here:
| 1. | \({P}_1<{P}_2\) |
| 2. | \({P}_1>{P}_2\) |
| 3. | \({P}_1={P}_2\) |
| 4. | Pressures can’t be related |
At room temperature the rms speed of the molecules of a certain diatomic gas is found to be 1930 m/s. The gas is
1.
2.
3.
4.
The latent heat of vaporization of water is 2240 J/g. If the work done in the process of vaporization of 1 gm is 168 J, then increase in internal energy is
1. 2408 J
2. 2240 J
3. 2072 J
4. 1904 J
For a gas, = 1.286. What is the number of degrees of freedom of the molecules of this gas ?
1. 3
2. 5
3. 6
4. 7
An ideal gas ( = 1.5) is expanded adiabatically. How many times has the gas to be expanded to reduce the root mean square velocity of molecules 2.0 times ?
1. 4 times
2. 16 times
3. 8 times
4. 2 times
A thin copper wire of length L increases in length by 1% when heated from to 0C to 100C . If a thin copper plate of area A is heated from to 0C to 100C, the percentage increase in its area will be
1. 1%
2. 2%
3. 3%
4. 4%