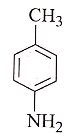
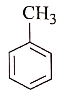
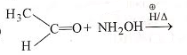In the reaction 
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The products of hydrolysis of
(1) HOCH2CH2CH2CH2 CHO + CH3CHO
(2) HOCH2CH2CH2CH2OH + CH3CHO
(3) HOCH2CH2CH2CH2CHO + C2H5OH
(4) HOCH2CH2CH2CH2CH2OH + C2H5OH
The product A is :
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
The major end product of the following reaction is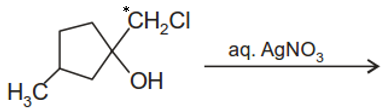
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Z
The product in the given reaction is
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
Bromination of (E)-2-butenedioic acid gives
(1) (2R, 3S)-2, 3-dibromosuccinic acid
(2) (2R, 3R)-2, 3-dibroniosuccinic acid
(3) a mixture of (2R, 3R) and (2S, 3S)-2, 3-dibromosuccinic acid
(4) (2S, 3S)-2, 3-dibromosuccinic acid
Consider the following reaction:-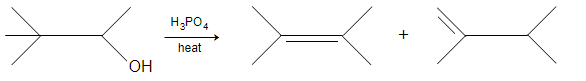
Which response contains all the correct statement about this process?
(1)Dehydration
(2)E2 mechanism
(3)Carbon skeleton migration
(4)Most stable alkene will form
(5)Single-step reaction
(a)1,3 (b)1,2,3 (c)1,2,5 (d)1,3,4
Which one of the following undergoes nucleophilic aromatic substitution at the fastest rate?
(1)
(2)
(3)
(4)
In E2 elimination, some compounds follow Hofmann's rule which means:
1. the double bond goes to the most substituted carbon
2. the compound is resistant to elimination
3. no double bond is formed
4. the double bond goes mainly towards the least substituted carbon
Heterolysis of propane gives:
(1) Methyl and ethyl free radicals
(2) Methylium cation and Ethyl anion
(3) Methyl anion and Ethylium cation
(4) Methylium and Ethylium cations
Carbanions initiate:
(1) addition reactions
(2) substitution reaction
(3) both (1) and (2)
(4) none of these
Ease of abstraction of hydrogen is greater when attached to:
(1) 1 carbon
(2) 2 carbon
(3) 3carbon
(4) neo carbon
SN1 mechanism for the reaction,
R-X+KOHROH+KX follow:
(1) carbocation mechanism
(2) carbanion mechanism
(3) free radical mechanism
(4) either of these
The formation of cyanohydrin from a ketone is an example of:
(1) electrophilic addition
(2) nucleophilic addition
(3) nucleophilic substitution
(4) electrophilic substitution
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S, the rates of the reaction vary as: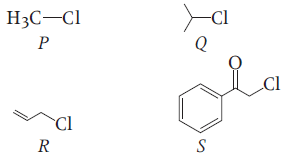
1. P > Q > R > S
2. S > P > R > Q
3. P > R > Q > S
4. R > P > S > Q
In an SN1 reaction on chiral centres there is
1. 100% racemisation
2. inversion more than retention leading to partial racemisation
3. 100% retention
4. 100% inversion
Consider the following reaction :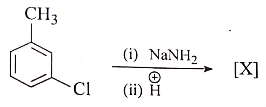
[X] will be :
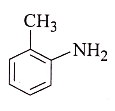
1. 
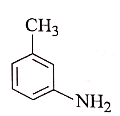
2. 
3. 
4. 
Match the List-I (Substrate and Reagent) with List-II (Product of the reaction) and select the correct answer from the codes given below the lists:
List-I :
(A) 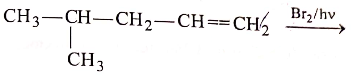
(B) 
(C) 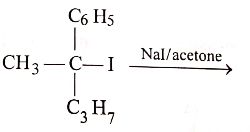
(D) 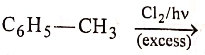
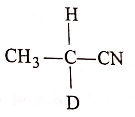
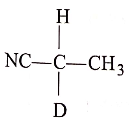
List-II :
(1) 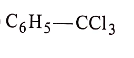
(2) 
(3) 
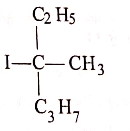
(4) 
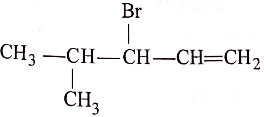
(5) 
Codes :
A B C D
1. 5 3 4 1
2. 5 2 4 1
3. 5 2 1 4
4. 5 2 3 4
Which one of the following is correctly matched?
1. 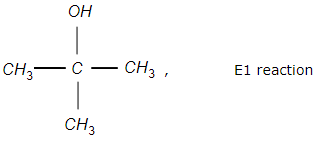
2. 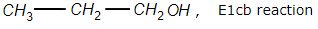
3.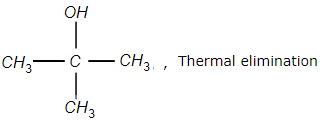
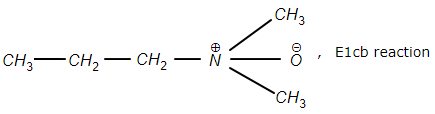
4. 
Arrange the reactivity of alcohols in decreasing order for E1 elimination reaction:
| I |  |
| II |  |
| III |  |
| IV |
Select the correct answer from the codes given below :
Codes :
1. III > II > IV > I
2. II > III > IV > I
3. II > III > I > IV
4. III > II > I > IV
In the given reaction, 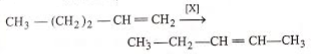
[x] will be :
1.
2.
3.
4.
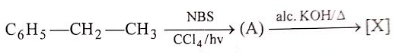 In the given reaction (A) and [X] respectively are :
In the given reaction (A) and [X] respectively are :
1.
2.
3.
4.
Which of the following reagents gives vic. diol with alkene?
1.
2.
3.
4. all of these
In the given reaction, 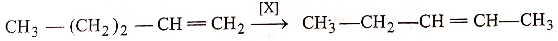
[X] will be :
1. anhy.
2. anhy.
3.
4. all of these
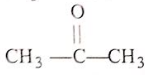
Arrange the reactivity of given compounds in decreasing order for addition reaction :
(1) 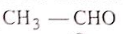
(2) 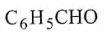
(3) 
(4) 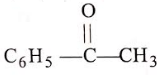
Select the correct answer freom the codes given below :
Codes :
1. 1, 2, 3, 4
2. 1, 2, 4, 3
3. 2, 1, 4, 3
4. 2, 1, 3, 4
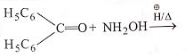
The product of which reaction will show geometrical isomerism?
1. 
2. 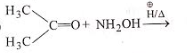
3. 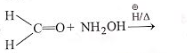
4.