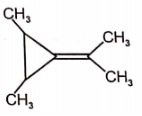
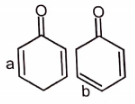
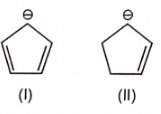
The most stable canonical structure among the given structures is/are :
| 1. | I | 2. | II |
| 3. | III | 4. | All are equally stable |
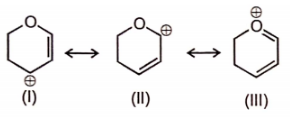
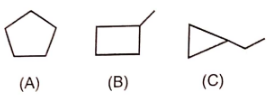
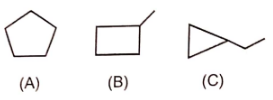
Which is not an example of resonance ?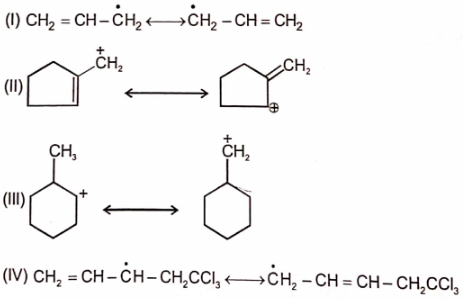
1. I
2. II
3. III
4. IV
The total number of resonating structures (excluded the given structure) formed by the given molecule are :
| 1. | 2 | 2. | 3 |
| 3. | 4 | 4. | 5 |
The carbocation among the following that doesn't get stabilized by resonance is :
| 1. | 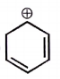 |
2. | 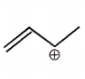 |
| 3. | 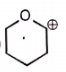 |
4. | 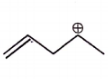 |
X= number of Resonance Structures contributed in Resonance Hybrid of 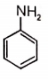
Y= number of Resonance Structures contributed in Resonance Hybrod of 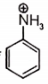
Find the sum of X + Y
1. 5
2. 6
3. 7
4. 8
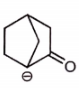
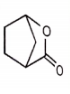
Which of the following compound is not resonance stablized ?
1. 
2. 
3. 
4. 
Number of o/p-directing groups present on benzene ring
(a) (b) (c) (d)
(e) 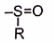 (f) (g) (h)
(f) (g) (h)
(i)
1. 5
2. 6
3. 7
4. 8
Which of the following pairs of structures do not represent resonance forms?
| 1. |  |
| 2. | 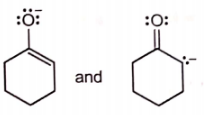 |
| 3. | 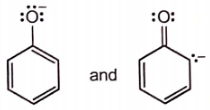 |
| 4. | 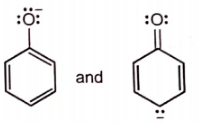 |
What is the type of conjugation of the given reaction?
1. Hyperconjugation
2. Simple Conjugation
3. Homocomjugation
4. Double hyperconjugation
Number of -p conjugation in the given compound is:
1. 4
2. 6
3. 7
4. 8
Compare the bond lengths of the indicated bonds in the given compound:
1. a > b
2. a < b
3. a = b
4. Cannot be predicted
Compare the bond lengths of the indicated bonds in the given compound:

1. a > b
2. a < b
3. a = b
4. Cannot be predicted
Compare the bond lengths of the indicated bonds in the given compound:
1. a > c > b
2. c > a > b
3. b > c > a
4. b > a > c
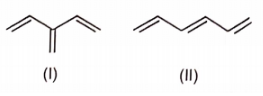
Correct order for the heat of combustion of the following is
(I) 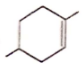 (II)
(II) 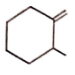 (III)
(III) 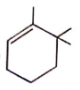 (IV)
(IV) 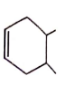
1. I > II > IV > III
2. IV > I > II > III
3. III > I > IV > II
4. III > II > IV > I
Which of the following wil have largest heat of combustion ?
1. A
2. B
3. C
4. All will have same heat of combustion because DBE = 1 for all.
Which of the given is most stable ?
1. A
2. B
3. C
4. All will have same heat of combustion because DBE = 1 for all.
In which of the following pair Ist compound has more resonance energy than IInd ?
1. 
2. 
3. 
4. 
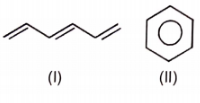
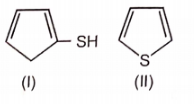
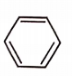
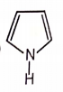
Which of the following aromatic compounds is the most stable ?
1. 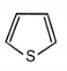
2. 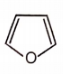
3. 
4. 
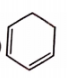
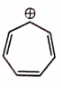
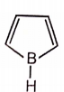
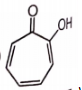
Which of the following is unstable at room temperature ?
1. 
2. 
3. 
4. 
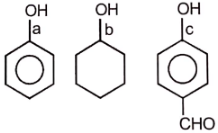
How many alcohols are possible for the molecular formula ( consider only structural isomers)
1. 6
2. 7
3. 8
4. 9
How many different structural isomers of molecular formula are possible, which contains five-membered Parent Chain?
1. 4
2. 5
3. 6
4. 7
How many structurally different alkynes are formed having molecular formula .
1. 2
2. 3
3. 4
4. 5
What are the number of structural isomers possible in 1-butene and 1,3-butadiene one H is replaced by D
1. 2, 0
2. 4, 2
3. 2, 4
4. 4, 4
Position isomers of 1-bromo-1-chloro cyclohexane are :
1. 6
2. 5
3. 4
4. 3
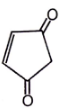
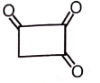
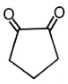
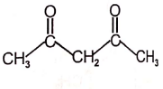
Which of the following compounds has negligible enol?
1. 
2. 
3. 
4. 
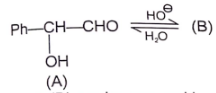
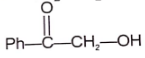
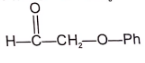
(A) & (B) are isomer and isomerization is effectively carried out by trace of base. Identify isomer (B).
1. 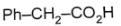
2. 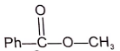
3. 
4. 
In the Newman projection of 2,3-dimethylpentane, which of the following options correctly identifies groups (A) and (B)?
1. \(- C H_{3} , - C H_{3}\)
2. \(- H , - C_{2} H_{5}\)
3. \(- C_{2} H_{5} , - C H_{3}\)
4. \(- C_{2} H_{5} , - C_{2} H_{5}\)
Rank the following conformations in order of increasing energy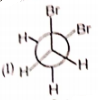
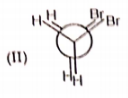
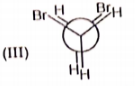
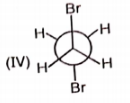
1. IV < I < III < II
2. III < II < IV < I
3. II < III < I < IV
4. IV < III < II < I
Dipole moment ofcis- 1, 2-dichloro ethene is :
1. 3
2.
3.
4. 2
When methyl group is in axial position in methyl cyclohexane, the molecule has :
1. One Gauche interaction
2. Two Gauche interaction
3. No Gauche interaction
4. Three Gauche interaction
Bridge head hydrogen of the conformer of cis-decaline is positioned as:
1. a, a
2. e, e
3. a, e
4. pseudo-a, pseudo-e
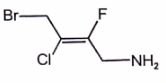
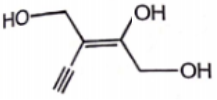
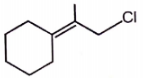
Which alkene has the Z configuration along '=' bond
1. 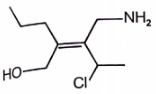
2. 
3. 
4. 
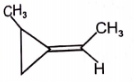
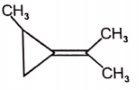
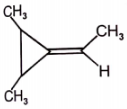
Among the following compounds, which can't show geometrical isomerism?
1. 
2. 
3. 
4.