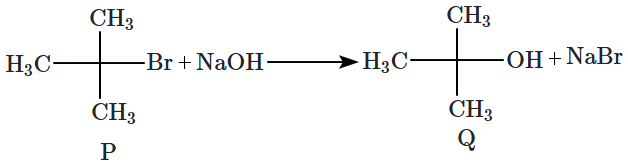1. The following data pertaining to the reaction between A and B is given with some inferences:
| S.No |
[A]
\(\mathrm{mol} . \mathrm{L}^{-1}\) |
[B]
\(\mathrm{mol} . \mathrm{L}^{-1}\) |
Rate
\(\mathrm{mol} . \mathrm{L}^{-1} \mathrm{sec}^{-1}\) |
| I |
\(1 \times 10^{-2}\) |
\(2 \times 10^{-2}\) |
\(2 \times 10^{-4}\) |
| II |
\(2 \times 10^{-2}\) |
\(2 \times 10^{-2}\) |
\(4 \times 10^{-4}\) |
| III |
\(2 \times 10^{-2}\) |
\(4 \times 10^{-2}\) |
\(8 \times 10^{-4}\) |
| a. |
The rate constant of the reaction is \(10^{-4}\). |
| b. |
The rate law of the reaction is \(k[A][B]\). |
| c. |
The rate of the reaction increases four times on doubling the concentration of both the reactants. |
Mark the correct option among the following:
| 1. |
a, b and c |
2. |
a and b |
| 3. |
b and c |
4. |
c alone |
2. The rate law for the dimerisation of \(NO_2\) is:
\(-\frac{\mathrm{d}\left[\mathrm{NO}_2\right]}{\mathrm{dt}}=\mathrm{k}\left[\mathrm{NO}_2\right]^2\)
The value of the specific rate constant, k can be changed by:
1. Doubling the total pressure on the system.
2. Doubling the temperature.
3. Both of (1) and (2)
4. None of the above.
3. For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. The average rate of the reaction in M sec–1 is:
1. 6.67×10–5 M s–1
2. 5.67×10–5 M s–1
3. 6.67×10–6 M s–1
4. 5.67×10–6 M s–1
4. \(C(s) + 2 H_2(g) \rightarrow CH_4(g); \Delta H = -74.8~kJ mol^{-1}\)
Which of the following diagrams gives an accurate representation of the above reaction?
[R→reactants; P→products]
5. Effective collisions are known to possess:
A: Energy greater than the threshold energy.
B: Breaking of old bonds in the reactant.
C: Formation of a new bond in the product.
D: High activation energy.
E: Proper orientation.
Choose the correct answer from the options given below:
| 1. |
A, B, C, D only |
2. |
A, B, C, E only |
| 3. |
A, C, D, E only |
4. |
B, C, D, E only |
6. The rate of a reaction doubles when its temperature changes from 300 K to 310 K. Activation energy of the reaction will be :
(R = 8.314 JK–1 mol–1 and log 2 = 0.301)
1. 53.6 kJ mol–1
2. 48.6 kJ mol–1
3. 58.5 kJ mol–1
4. 60.5 kJ mol–1
7. If the rate constant of a reaction is
\(0.03 s^{-1}\), how much time does it take for
\(7.2\text { mol L}^{-1}\) concentration of the reactant to get reduced to
\(0.9~\text {mol L} ^{-1}?\)
(Given: log 2=0.301)
| 1. |
210 s |
2. |
21.0 s |
| 3. |
69.3 s |
4. |
23.1 s |
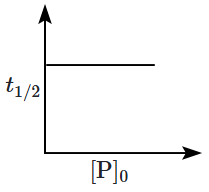
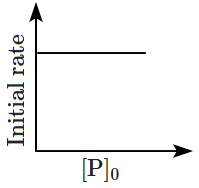
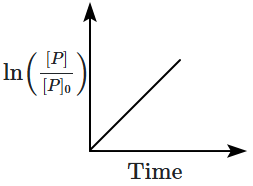
8. Which of the following plots is correct for the given reaction?
([P]
0 is the initial concentration of P)
9. The order of a reaction, given that the initial concentration of 0.24 M is reduced to 0.12 M in 10 hours and to 0.06 M in 20 hours, is:
| 1. |
Zero order reaction |
2. |
First order reaction |
| 3. |
Second order reaction |
4. |
Half order reaction |
10.
| Statement I: |
Order is applicable only for elementary reactions whereas molecularity is applicable for both elementary as well as complex reactions. |
| Statement II: |
Order of a reaction is an experimental quantity. |
In the light of the above statements, identify the correct option:
1. Both
Statement I and
Statement II are correct
2. Both
Statement I and
Statement II are incorrect
3.
Statement I is incorrect but
Statement II is correct
4.
Statement I is correct but
Statement II is incorrect
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course