Physics
1. The diameter of a thin wire is measured by:
| 1. |
Screw gauge |
2. |
Spherometer |
| 3. |
Spectrometer |
4. |
Venturimeter |
2. The dimensions of momentum per unit time is the same as that of:
| 1. |
Torque / Mass |
2. |
Energy / Length |
| 3. |
Acceleration / Mass |
4. |
Power / Acceleration |
3. What is the unit of the quantity represented by: \(\dfrac{\text{(Angular momentum)}}{\text{(electric charge)}^2}\)
1. \(\Omega\) (ohm)
2. s/m
3. \(\text{H }\)(henry)
4. \(\text{F }\) (farad)
4. A physical quantity of the dimensions of length that can be formed out of \(c, G,~\text{and}~\dfrac{e^2}{4\pi\varepsilon_0}\)is:
(\(c\) is the velocity of light, \(G\) is the universal constant of gravitation and \(e\) is charge)
| 1. |
\(c^2\left[G \dfrac{e^2}{4 \pi \varepsilon_0}\right]^{\dfrac{1}{2}}\) |
2. |
\(\dfrac{1}{c^2}\left[\dfrac{e^2}{4 G \pi \varepsilon_0}\right]^{\dfrac{1}{2}}\) |
| 3. |
\(\dfrac{1}{c} G \dfrac{e^2}{4 \pi \varepsilon_0}\) |
4. |
\(\dfrac{1}{c^2}\left[G \dfrac{e^2}{4 \pi \varepsilon_0}\right]^{\dfrac{1}{2}}\) |
5. The angular momentum of a rotating sphere of mass-\(m,\) radius-\(r\) is computed from the expression: \(L=\dfrac25mr^2\omega,\) where \(\omega\) is the angular speed of rotation. The mass is known to within \(0.5\%,\) the radius to \(0.5\%,\) and the angular speed \((\omega)\) to within \(1\%.\) The fractional error in \(L\) is:
1. \(1\%\)
2. \(1.5\%\)
3. \(2\%\)
4. \(2.5\%\)
6. Among the following numerical values, which one has three significant figures?
1. \(0.300\)
2. \(30.30\)
3. \(0.030\)
4. \(3.033\)
7. Express the result of the calculation to
\(3\) significant figures:
\(2\times0.536+0.0050+2.100\)
| 1. |
\(3.177\) |
2. |
\(3.18\) |
| 3. |
\(3.19\) |
4. |
\(3.2\) |
8. The dimensions \([MLT^{-2}A^{-2}]\) belong to the:
1. electric permittivity
2. magnetic flux
3. self-inductance
4. magnetic permeability
9. If dimensions of critical velocity \({v_c}\) of a liquid flowing through a tube are expressed as \(\eta^{x}\rho^yr^{z}\), where \(\eta, \rho~\text{and}~r\) are the coefficient of viscosity of the liquid, the density of the liquid, and the radius of the tube respectively, then the values of \({x},\) \({y},\) and \({z},\) respectively, will be:
| 1. |
\(1,-1,-1\) |
2. |
\(-1,-1,1\) |
| 3. |
\(-1,-1,-1\) |
4. |
\(1,1,1\) |
10. The errors in the measurement which arise due to unpredictable fluctuations in temperature and voltage supply are:
| 1. |
Random errors |
2. |
Instrumental errors |
| 3. |
Personal errors |
4. |
Least count errors |
11. The force
\(F\) acting on a body as a function of position
\((x)\) and time
\((t)\) is expressed as
\(F=A \sin \left(k_1 x\right)+B \cos \left(k_2 t\right).\) From the given information, match
Column-I with
Column-II.
|
Column-I |
|
Column-II |
| \((\mathrm A)\) |
Dimensions of \(A\) |
\((\mathrm P)\) |
\([M^0L^0T^{-1}]\) |
| \((\mathrm B)\) |
Dimensions of \(k_{1}\) |
\((\mathrm Q)\) |
\([M^0L^{-1}T^{-1}]\) |
| \((\mathrm C)\) |
Dimensions of \(k_{2}\) |
\((\mathrm R)\) |
\([MLT^{-2}]\) |
| \((\mathrm D)\) |
Dimensions of \(k_{1}k_{2}\) |
\((\mathrm S)\) |
\([M^0L^{-1}T^{0}]\) |
Codes:
| 1. |
\(\mathrm {A \rightarrow R, B \rightarrow S, C \rightarrow P, D \rightarrow Q }\) |
| 2. |
\(\mathrm {A \rightarrow P, B \rightarrow Q, C \rightarrow R, D \rightarrow S }\) |
| 3. |
\(\mathrm {A \rightarrow R, B \rightarrow P, C \rightarrow Q, D \rightarrow S }\) |
| 4. |
\(\mathrm {A \rightarrow S, B \rightarrow P, C \rightarrow Q, D \rightarrow R}\) |
12. If the unit of length is the distance between the earth and the sun, and the unit of time is
\(1\) year, then the speed of the earth around the sun is numerically equal to:
| 1. |
\(1\) |
2. |
\(2\) |
| 3. |
\(2\pi\) |
4. |
\(\dfrac1{2\pi}\) |
13. What is the maximum percentage error in the measurement of the quantity
\(I=\dfrac{a^2 b^3}{c \sqrt{d}},\)
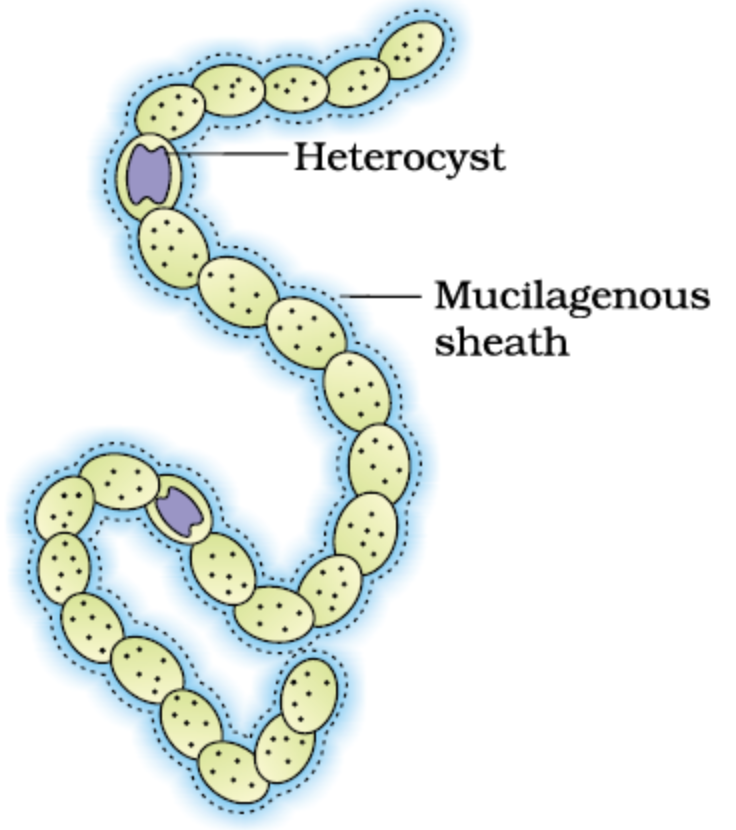
if the percentage errors in the measurements of
\(a, b,c,\) and
\(d\) are
\(1\text{%},\) \(2\text{%},\) \(3\text{%},\) and
\(4\text{%},\) respectively?
| 1. |
\(11\text{%}\) |
2. |
\(12\text{%}\) |
| 3. |
\(9\text{%}\) |
4. |
\(13\text{%}\) |
14. Match List- I with List- II.
|
List-I
(Measured values) |
|
List-II
(Significant figures) |
| A. |
\(0.001213\) |
I. |
\(2\) |
| B. |
\(2.1 \times 10^{16} \) |
II. |
\(3\) |
| C. |
\(3.70\) |
III. |
\(1\) |
| D. |
\(3000\) |
IV. |
\(4\) |
Choose the correct answer from the options given below:
| 1. |
A-III, B-II, C-I, D-IV |
2. |
A-III, B-I, CII, D-IV |
| 3. |
A-I, B-II, C-IV, D-III |
4. |
A-IV, B-I, C-II, D-III |
15. The quantities
\(K,c,\eta \) represent the thermal conductivity, the specific heat capacity and the viscosity of a liquid. Which of the following, is dimensionless?
1.
\(Kc\eta\)
2.
\(\dfrac{Kc}{\eta}\)
3.
\(\dfrac{K\eta}{c}\)
4.
\(\dfrac{\eta c}{K}\)
16. The diameter of a spherical bob, when measured with vernier callipers yielded the values: \(3.33\) cm, \(3.32\) cm, \(3.34\) cm, \(3.33\) cm and \(3.32\) cm. The mean diameter to appropriate significant figures is:
1. \(3.328\) cm
2. \(3.3\) cm
3. \(3.33\) cm
4. \(3.32\) cm
17. A screw gauge gives the following readings when used to measure the diameter of a wire:
Main scale reading: \(0\) mm
Circular scale reading: \(52\) divisions
Given that \(1\) mm on the main scale corresponds to \(100\) divisions on the circular scale, the diameter of the wire that can be inferred from the given data is:
| 1. |
\(0.26\) cm |
2. |
\(0.052\) cm |
| 3. |
\(0.52\) cm |
4. |
\(0.026\) cm |
18. Plane angle and solid angle have:
| 1. |
both units and dimensions |
| 2. |
units but no dimensions |
| 3. |
dimensions but no units |
| 4. |
no units and no dimensions |
19. In a vernier callipers,
\((N+1)\) divisions of the vernier scale coincide with
\(N\) divisions of the main scale. If
\(1\) \(\text{MSD}\) represents
\(0.1~\text{mm}\), the vernier constant (in
\(\text{cm}\)) is:
| 1. |
\(\dfrac{1}{100(N+1)} \) |
2. |
\(100N\) |
| 3. |
\(10(N+1) \) |
4. |
\(\dfrac{1}{10 N}\) |
20. If the error in the measurement of mass is \(0.8\%\) and in volume it is \(0.4 \%,\) then the error in the measurement of density is:
1. \(1.2 \%\)
2. \(0.4 \%\)
3. \(0.8\%\)
4. \(1 \%\)
21. Given below are two statements:
| Statement I: |
All dimensionless quantities must be constants. |
| Statement II: |
Two quantities having the same dimensions must be proportional to each other. |
| 1. |
Statement I is incorrect and Statement II is correct. |
| 2. |
Both Statement I and Statement II are correct. |
| 3. |
Both Statement I and Statement II are incorrect. |
| 4. |
Statement I is correct and Statement II is incorrect. |
22. The length of a simple pendulum is measured with an error of \(0.1\%,\) and its time period is measured with an error of \(2\%.\) If a physical quantity has the dimensional formula \(\left [ LT^{-1} \right ],\) the maximum possible percentage error in its measurement is:
| 1. |
\(1.1\%\) |
2. |
\(2.1\%\) |
| 3. |
\(4.1\%\) |
4. |
\(6.1\%\) |
23. Time intervals measured by a clock give the following readings:
\(1.25~\text{s},~1.24~\text{s}, ~1.27~\text{s},~1.21~\text{s},~1.28~\text{s}.\)
What is the percentage relative error of the observations?
1. \(2\)%
2. \(4\)%
3. \(16\)%
4. \(1.6\)%
24. Dimensions of stress are:
| 1. |
\( {\left[{ML}^2 {T}^{-2}\right]} \) |
2. |
\( {\left[{ML}^0 {T}^{-2}\right]} \) |
| 3. |
\( {\left[{ML}^{-1} {T}^{-2}\right]} \) |
4. |
\( {\left[{MLT}^{-2}\right]}\) |
25. The pitch of an error-free screw gauge is
\(1~\text{mm},\) and there are
\(100\) divisions on the circular scale. While measuring the diameter of a thick wire, the pitch scale reads
\(1~\text{mm},\) and
\(63^\text{rd}\) division on the circular scale coincides with the reference line. The diameter of the wire is:
| 1. |
\(1.63 ~\text{cm}\) |
2. |
\(0.163 ~\text{cm}\) |
| 3. |
\(0.163~\text m\) |
4. |
\(1.63 ~\text m\) |
26. The estimate of absolute error in the measurement of time using a clock is \(10^{-2}~\text{s}.\) The time difference, \(t=t_1-t_2,\) between two events is determined by using the clock. The error in \(t\) is:
1. \(10^{-2}~\text{s}\)
2. \(2\times10^{-2}~\text{s}\)
3. \(\dfrac{1}{2}\times10^{-2}~\text{s}\)
4. zero
27. The dimension of which of the quantities is the same as that of electrical inductance?
| 1. |
\(\dfrac{\text{Resistance}}{\text{Capacitance}}\) |
| 2. |
\({\text{Resistance}}\times{\text{Capacitance}}\) |
| 3. |
\(\dfrac{\text{Capacitance}}{\text{Resistance}}\) |
| 4. |
\({\text{(Resistance)}}^2\times{\text{Capacitance}}\) |
28. Taking into account the significant figures, what is the value of
\((9.99~\text{m}-0.0099~\text{m})\)?
| 1. |
\(9.98~\text{m}\) |
2. |
\(9.980~\text{m}\) |
| 3. |
\(9.9~\text{m}\) |
4. |
\(9.9801~\text{m}\) |
29. The mechanical quantity, which has dimensions of reciprocal of mass
\((M^{-1}),\) is:
| 1. |
angular momentum |
| 2. |
coefficient of thermal conductivity |
| 3. |
torque |
| 4. |
gravitational constant |
30. The unit of energy is
\(e\text {V}\) and the unit of velocity is
\(c,\) in a certain system. The unit of momentum in this system is:
| 1. |
\(e\text V\times c \) |
2. |
\(\Large\frac{e\text V}{c} \) |
| 3. |
\(\Large\frac{e\text V}{c^2} \) |
4. |
\(e\text V\times c^2 \) |
31. The area of a rectangular field (in \(\text{m}^2\)) of length \(55.3\) m and breadth \(25\) m after rounding off the value, for correct significant digits is:
1. \(14\times10^{2}\)
2. \(138\times10^{1}\)
3. \(1382\)
4. \(1382.5\)
32. The least count of a stopwatch is \(0.1\) sec. The time of \(20\) oscillations of the pendulum is found to be \(20\) sec. The percentage error in the time period is:
1. \(0.25\%\)
2. \(0.5\%\)
3. \(0.75\%\)
4. \(1.0\%\)
33. Match the following quantities in
List-I with their dimensions in
List-II.
| List-I |
List-II |
| (a) |
acceleration |
(i) |
\([M^0L^0T^0 ]\) |
| (b) |
torque |
(ii) |
\([ML^{-1}T^{-2} ]\) |
| (c) |
absorptive power |
(iii) |
\([LT^{-2} ]\) |
| (d) |
pressure |
(iv) |
\([ML^2T^{-2} ]\) |
| 1. |
(a)-(iii), (b)-(iv), (c)-(i), (d)-(ii) |
| 2. |
(a)-(iii), (b)-(ii), (c)-(i), (d)-(iv) |
| 3. |
(a)-(iii), (b)-(i), (c)-(ii), (d)-(iv) |
| 4. |
(a)-(ii), (b)-(iv), (c)-(iii), (d)-(i) |
34. Suppose that the value of Newton's gravitational constant is determined from the equation;
\(F=G{\Large\frac{m_1m_2}{r^2}},\) by a taking direct measurement of the quantities. The relative errors in the measurements are: force,
\(F\rightarrow2\%,\) masses
\((m_1,m_2)\rightarrow1\%\) and separation
\((r)\rightarrow1\%.\) The relative error in the measurement of
\(G\) will be:
| 1. |
\(2\%\) |
2. |
\(4\%\) |
| 3. |
\(6\%\) |
4. |
\(8\%\) |
35. The velocity \(v\) of a particle at a time \(t\) is given by \(v=a t+\dfrac{b}{t+c}.\) The dimensions of \(a,\) \(b\) and \(c\) are respectively:
1. \(\left [ LT^{-2} \right ],\) \(\left [ L\right ],\) \(\left [ T\right ]\)
2. \(\left [ L^{2}\right ],\) \(\left [ T\right ],\) \(\left [ LT^{2} \right ]\)
3. \(\left [ LT^{2} \right ],\) \(\left [ LT \right ],\) \(\left [ L\right ]\)
4. \(\left [ L\right ],\) \(\left [ LT \right ],\) \(\left [ T^{2}\right ]\)
36. The error in the measurement of distance is
\(2\%,\) while that in time is
\(1\%.\) The error in speed, computed from the above, is:
| 1. |
\(2\%+1\%\) |
2. |
\(2\%-1\%\) |
| 3. |
\(\dfrac{2\%+1\%}{2}\) |
4. |
\(\dfrac{2\%-1\%}{2}\) |
37. The percentage errors in the measurement of mass and momentum of an object are
\(1\%\) and
\(2\%\) respectively. The percentage error in the measurement of kinetic energy of the object will be:
| 1. |
\(1\%\) |
2. |
\(3\%\) |
| 3. |
\(4\%\) |
4. |
\(5\%\) |
38. Given that
\(T\) stands for time period and
\(l\) stands for the length of simple pendulum. If
\(g\) is the acceleration due to gravity, then which of the following statements about the relation
\(T^{2}~=l/g\) is correct?
| 1. |
It is correct both dimensionally as well as numerically. |
| 2. |
It is neither dimensionally correct nor numerically. |
| 3. |
It is dimensionally correct but not numerically. |
| 4. |
It is numerically correct but not dimensionally. |
39. Which of the following, is dimensionless?
| 1. |
\(\dfrac{\text{pressure}}{\text{density}\times\text{acceleration}}\) |
2. |
\(\dfrac{\text{density}\times\text{pressure}}{\text{speed}}\) |
| 3. |
\(\dfrac{\text{viscosity}\times\text{speed}}{\text{surface tension}}\) |
4. |
\(\dfrac{\text{surface tension}}{\text{(speed)}^2}\) |
40. In an experiment, the percentage errors that occurred in the measurement of physical quantities \(A,\) \(B,\) \(C,\) and \(D\) are \(1\%\), \(2\%\), \(3\%\), and \(4\%\) respectively. Then, the maximum percentage of error in the measurement of \(X,\) where \(X=\frac{A^2 B^{\frac{1}{2}}}{C^{\frac{1}{3}} D^3}\), will be:
| 1. |
\(10\%\) |
2. |
\(\dfrac{3}{13}\%\) |
| 3. |
\(16\%\) |
4. |
\(-10\%\) |
41. Match the following quantities in
List I with their dimensions in
List II:
| List I |
List II |
| (P) |
Young's modulus |
(A) |
\([ML^2T^{-1}]\) |
| (Q) |
Planck's constant |
(B) |
\([ML^{-1}T^{-2}]\) |
| (R) |
Work function |
(C) |
\([ML^{-1}T^{-1}]\) |
| (S) |
Coefficient of viscosity |
(D) |
\([ML^2T^{-2}]\) |
Codes:
| 1. |
P → A, Q → B, R → C, S → D |
| 2. |
P → B, Q → A, R → D, S → C |
| 3. |
P → D, Q → A, R → C, S → B |
| 4. |
P → D, Q → A, R → B, S → C |
42. The radius of a ball is
\(\left({{5}{.}{2}\pm{0}{.}{2}}\right)\) cm. The percentage error in the volume of the ball is:
| 1. |
\(11 \text{%}\) |
2. |
\(4 \text{%}\) |
| 3. |
\(7 \text{%}\) |
4. |
\(9 \text{%}\) |
43. The unit of thermal conductivity is:
| 1. |
W m–1 K–1 |
2. |
J m K–1 |
| 3. |
J m–1 K–1 |
4. |
W m K–1 |
44. If force (\(F\)), velocity (\(\mathrm{v}\)), and time (\(T\)) are taken as fundamental units, the dimensions of mass will be:
| 1. |
\([FvT^{-1}]\) |
2. |
\([FvT^{-2}]\) |
| 3. |
\([Fv^{-1}T^{-1}]\) |
4. |
\([Fv^{-1}T]\) |
45. The energy required to break one bond in DNA is \(10^{-20}~\text{J}\). This value in eV is nearly:
1. \(0.6\)
2. \(0.06\)
3. \(0.006\)
4. \(6\)
Chemistry
46. The mass of H
2O formed by the reaction of 11.2 L H
2 and excess O
2 at STP will be:
1. 18 g
2. 9 g
3. 36 g
4. 4.5 g
47. Determine the empirical and molecular formulae of a compound given the following percentage composition by mass:
4.07% hydrogen, 24.27% carbon, and 71.65% chlorine, with a molar mass of 98.96 g/mol.
1. CH2Cl and C2H4Cl2
2. CH3Cl and C2H6Cl2
3. C2HCl and C4H2Cl2
4. C2HCl and C2H4Cl2
48. The number of moles of carbon and oxygen required to produce \(6.023 \times 10^{23}\) molecules of \(CO_2\) are:
1. 0.01 mol and 0.01 mol
2. 0.5 mol and 0.1 mol
3. 10.0 mol and 5.0 mol
4. 1.0 mol and 1.0 mol
49. Match List-I with List-II:
|
List-I
(Quantities) |
|
List-II
(Corresponding Values) |
| (a) |
4.48 litres of O2 at STP |
(i) |
0.2 mole |
| (b) |
12.022 × 1022 molecules of H2O |
(ii) |
12.044 × 1023
molecules |
| (c) |
96 g of O2 |
(iii) |
6.4 g |
| (d) |
88 g of CO2 |
(iv) |
67.2 litres at STP |
(Given - Molar volume of a gas at STP - 22.4 L)
Choose the correct answer from the options given below:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(i) |
(iii) |
(iv) |
(ii) |
| 2. |
(iii) |
(i) |
(iv) |
(ii) |
| 3. |
(iv) |
(i) |
(ii) |
(iii) |
| 4. |
(iii) |
(i) |
(ii) |
(iv) |
50. How much glucose is needed to prepare 250 mL of a 1/20 M (M/20) glucose solution?
(Molar mass of glucose: 180 g/mol)
1. 2.25 g
2. 4.5 g
3. 0.44 g
4. 1.125 g
51. What is the molality of a solution containing 20% (mass/mass) KI in water?
(molar mass of KI = 166 g mol–1)
1. 1.51
2. 7.35
3. 4.08
4. 2.48
52. 10.6 gm \(Na_2CO_3 \) is dissolved in 250 ml \(H_2O \) . The molarity of \(Na^+ \) ions and \(CO^{2-}_3\) in this solution, respectively, are:
1. 0.4 M and 0.2 M
2. 0.8 M and 0.4 M
3. 0.4 M and 0.4 M
4. 0.4 M and 0.8 M
53. What would be the molarity of a solution of ethanol in water if the mole fraction of ethanol is 0.040?
(assume the density of water to be 1)
1. 3.143 M
2. 2.314 M
3. 2.413 M
4. 2.141 M
54. 10 gm carbon reacts with 100 gm \(Cl_2\) to form \(CCl_4.\) The maximum weight of \(CCl_4\) formed will be:
1. 128 gm
2. 94.22 gm
3. 108.45 gm
4. 54.22 gm
55. 0.8 mole of a mixture of CO and CO2 requires exactly 40 gram of NaOH in a solution for complete conversion of all the CO2 into Na2CO3. How many moles more of NaOH would it require for conversion into Na2CO3, if mixture (0.8 mole) is completely oxidised to CO2?
1. 0.2
2. 0.6
3. 1
4. 1.5
56. 1.00 g of hydrated potassium carbonate, K2CO3•nH2O, is heated to 250 ºC to give 0.836 g anhydrous K2CO3. What is the value of n?
1. 0.16
2. 1.0
3. 1.5
4. 2.0
57. Determine the percent yield of ammonia in the following reaction, where 0.25 mol of NH
3 is produced from the reaction of 0.5 mol of N
2 with 0.5 mol of H
2.
\(\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{NH}_3(\mathrm{~g})\)
| 1. |
75% |
2. |
50% |
| 3. |
33% |
4. |
25% |
58. What can be the empirical formula of a compound, if it contains 51.9% carbon, 4.86% hydrogen, and 43.2% bromine by mass?
| 1. |
\(\text{C}_7\text{H}_5\text{Br}\) |
2. |
\(\text{C}_6\text{H}_4\text{Br}_3\) |
| 3. |
\(\text{C}_8\text{H}_9\text{Br}\) |
4. |
\(\text{C}_{12}\text{H}_{22}\text{Br}\) |
59. Given below are two statements:
| Assertion (A): |
Both 106 g of sodium carbonate and 12 g of graphite have the same number of carbon atoms. |
| Reason (R): |
Both 106 g of sodium carbonate and 12 g of graphite contain 1 g atom (1 mole) of carbon atoms. |
| 1. |
Both (A) and (R) are True, and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True, but (R) is not the correct explanation of (A). |
| 3. |
(A) is True, but (R) is False. |
| 4. |
Both (A) and (R) are False. |
60. For the reaction,
\(2A+B\xrightarrow{~~~~~~~~}3C,8\) moles of
\(A\) reacts with
\(5\) moles of
\(B\) to form
\(C.\)
Calculate the maximum number of moles that are formed.
| 1. |
\(13\) mole |
2. |
\(15\) mole |
| 3. |
\(12\) mole |
4. |
\(8\) mole |
61. Number of atoms present in 5 g monoatomic gas with vapour density 8 will be:
(Vapour density = \(\frac{Molar \ mass}{2}\))
1. \(0.625N_A\)
2. \(0.25 N_A\)
3. \(0.3125N_A\)
4. \(N_A\)
62. The mass of one atom of 12C in grams is:
1. 1.99 x 10-23g
2. 22 x 10-23g
3. 6.022 x 1023g
4. 1.99 x 1023g
63. In the following reaction carried out at 500K, 18 moles of N2 gas is mixed with 24 moles of H2 gas. What is the percentage yield of NH3 if the reaction produces 13.5 moles of NH3?
N2(g) + 3 H2 (g) \(\rightarrow\) 2NH3 (g)
1. 16%
2. 66%
3. 72%
4. 84%
64. Which of the following compounds has the same empirical formula as that of glucose?
1. \(CH_3CHO\)
2. \(CH_3COOH\)
3. \(CH_3OH\)
4. \(C_2H_6\)
65. A sample of a pure compound was found to contain carbon, hydrogen, and chlorine.
Its elemental analysis is given below:
| Element |
Composition/Weight |
| Carbon |
1.201 grams |
| Hydrogen |
0.202 grams |
| Chlorine |
7.090 grams |
What is the empirical formula for the compound?
| 1. |
\(\mathrm {CHCl}_3\) |
2. |
\(\mathrm {CH}_2\mathrm {Cl}\) |
| 3. |
\(\mathrm {CH}_2\mathrm {Cl}_2\) |
4. |
\(\mathrm {CH}_3\mathrm {Cl}\) |
66. When 50.0 kg of nitrogen gas (N2) and 10.0 kg of hydrogen gas (H2) are combined, what is the quantity of ammonia gas (NH3) produced?
1. 33.0 ×103 mol
2. 56.1×103 g
3. 6.5 ×103 g
4. None of the above
67. The empirical formula of an organic compound containing carbon and hydrogen is CH2. The mass of one litre of this organic gas at STP is exactly equal to that of one litre of N2 at STP. Therefore, the molecular formula of the organic gas is:
1. C2H4
2. C3H6
3. C6H12
4. C4H8
68. How many significant figures are present in the following numbers?
| (I) |
161 cm |
| (II) |
0.0161 |
| (III) |
1.61 |
1. 3, 3, 3
2. 3, 4, 3
3. 3, 2, 3
4. 3, 4, 4
69. 6.02 × 10
20 molecules of urea are present in 100 mL of its solution. The concentration of the solution is:
| 1. |
0.01 M |
2. |
0.001 M |
| 3. |
0.1 M |
4. |
0.02 M |
70. A solution of phenol in chloroform when treated with aqueous NaOH gives compound P as a major product. The mass percentage of carbon in P is:
(to the nearest integer) (Atomic mass : C =12; H=1; O=16)
71. The number of moles of hydrogen gas liberated when 39 g of potassium reacts with 7.8 g of water is:
1. 0.22 mol
2. 0.43 mol
3. 0.50 mol
4. 1.0 mol
72. A compound contains 17.28 % nitrogen, and its molecular mass is 162. The number of nitrogen atoms present in one molecule of the alkaloid are:
| 1. |
Five |
2. |
Four |
| 3. |
Three |
4. |
Two |
73. How much volume of 75% alcohol by weight (d=0.80 g/cm³) is required to prepare 150 cm³ of 30% alcohol by weight (density = 0.90 g/cm³)?
| 1. |
67.5 mL |
2. |
56.25 mL |
| 3. |
44.44 mL |
4. |
None of the above |
74. The number of sodium atoms in 2 moles of sodium ferrocyanide are:
| 1. |
12×1023
|
2. |
26×1023
|
| 3. |
34×1023
|
4. |
48×1023 |
75. 2.5 litre mixture of CO and CO2 is passed through red hot charcoal in a tube. The new volume becomes 3.5 litre. All measurements are made at same temperature and pressure. Percentage composition of original mixture by volume will be:
1. CO = 50%, CO2 = 50%
2. CO = 60%, CO2 = 40%
3.CO = 40%, CO2 = 60%
4.CO = 20%, CO2 = 80%
76. 1 gram of a carbonate (M2CO3) on treatment with excess HCl produces 0.01186 moles of CO2. The molar mass of M2CO3 in g mol–1 is:
1. 118.6
2. 11.86
3. 88.6
4. 84.3
77. Carbon and oxygen combine to form two oxides, carbon monoxide and carbon dioxide in which the ratio of the weights of carbon and oxygen is respectively 12 : 16 and 12 : 32. These figures illustrate the:
1. Law of multiple proportions
2. Law of reciprocal proportions
3. Law of conservation of mass
4. Law of constant proportions
78. A hydrocarbon has a mass ratio of carbon to hydrogen of 6:2. What is the empirical formula of this hydrocarbon?
1. CH
2.
3.
4.
79. Calculate the approximate mass of
\(CO_2\) produced when 1 gm of butane
\((C_4H_{10})\) is burned with
an excess of
\(O_2\) to form
\(CO_2\).
| 1. |
1 g |
2. |
2 g |
| 3. |
3 g |
4. |
4 g |
80.
Which among the following has the greatest number of atoms at NTP?
| 1. |
5 ml CH4 |
2. |
20 ml N2 |
| 3. |
1 ml H2O |
4. |
10 ml CO2 |
81. The molarity of \(NaNO_3 \) solution (molecular weight of \(NaNO_3\) = 85) is 1M. The density of the solution is 1.25 gm/ml. The molality of the solution, approximately, will be:
1. 0.80
2. 0.86
3. 0.96
4. 1
82.
At constant T and P, 5.0 L of SO2 is reacted with 3.0 L of O2 according to the following equation:
2 SO2(g) + O2(g) → 2 SO3(g)
The volume of the remaining reaction mixture at the completion of the reaction will be:
| 1. |
0.5 L |
2. |
8.0 L |
| 3. |
5.5 L |
4. |
5 L |
83. 5 moles of AB2 weight kg and 10 moles A2B2 weight kg. The molar mass of A (MA) and molar mass of B (MB) in kg mol are:
| 1. |
MA = 10×10-3 and MB = 5×10-3 |
| 2. |
MA = 25×10-3 and MB = 50×10-3 |
| 3. |
MA = 5×10-3 and MB = 10×10-3 |
| 4. |
MA= 50×10-3 and MB = 25×10-3 |
84. \(6.02\times10^{20}\) molecules of urea are present in 100 mL of its solution. The concentration of urea solution is:
(Avogadro constant, \(N_A=6.02\times10^{23}~mol^{-1}\))
1. 0.001 M
2. 0.01 M
3. 0.02 M
4. 0.1 M
85. Which of the following statements is true for the solution of 0.020 M H2SO4?
1. 2 litres of the solution contains 0.020 moles of \(\mathrm{SO}_4^{2-}\)
2. 2 litres of the solution contains 0.080 moles of H3O+
3. 1 litre of the solution contains 0.020 moles H3O+
4. None of the above three statements is true
86. In the Haber process reaction, \(\text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3\) , starting with 5 L of \( \text{N}_2 \) at STP and 5 L of \(\text{H}_2 \), which of the following is the limiting reagent?
1. \( \text{N}_2 \)
2. \(\text{H}_2 \)
3. Both \( \text{N}_2 \) and \(\text{H}_2 \)
4. Neither \( \text{N}_2 \) nor \(\text{H}_2 \)
87. Calculate the mass of 95% pure CaCO
3 that will be required to neutralize
50 mL of
0.5 M HCl solution according to the following reaction.
CaCO
3(s) + 2HCl
(aq) → CaCl
2(aq) + CO
2(g) + 2H
2O
(l)
[Calculate up to the second place of decimal point]
| 1. |
9.50 g |
2. |
1.25 g |
| 3. |
1.32 g |
4. |
3.65 g |
88. At 120 ºC and 1 atm pressure, 1.00 L of methane reacts completely with excess oxygen to form carbon dioxide and water.
The volumes of the two products at this pressure and temperature are:
1. 1.00 L CO2 and 2.00 L H2O.
2. 1.00 L CO2 and 4.00 L H2O.
3. 2.00 L CO2 and 2.00 L H2O.
4. 2.00 L CO2 and 4.00 L H2O.
89. The weight of solid NaHCO3 (in grams) required to neutralize 40.0 mL of 0.1 M H2SO4 solution is:
1. 0.672 g
2. 6.07 g
3. 17 g
4. 20 g
90. Nitrogen occurs in the form of two isotopes with atomic masses 14 and 15, respectively. The average atomic mass of nitrogen is 14.0067. The % abundance of isotope with atomic mass 14 is:
1. 99.3 %
2. 86.4 %
3. 82.1 %
4. 92.2 %
Biology
91. After ovulation, the ruptured follicle is converted to a structure called corpus luteum, which secretes mainly:
1. Oestrogen
2. Progesterone
3. DHEA
4. Corticosterone
92. Normal sleep-wake cycle in a human body is maintained by the secretion of:
1. Thyroid gland
2. Thymus gland
3. Pineal gland
4. Pituitary gland
93. Identify the correct statements regarding diatoms:
| I: |
Diatoms are chief producers in the oceans |
| II: |
Diatoms are microscopic and float passively in water |
| III: |
The walls of diatoms are easily destructible |
| IV: |
'Diatomaceous earth' is formed by the cell walls of diatoms |
1. Only
I,
II and
III
2. Only
I,
II and
IV
3. Only
II,
III and
IV
4.
I,
II,
III and
IV
94. Which of the following is an accumulation and release centre of neurohormones?
| 1. |
Posterior pituitary lobe |
| 2. |
Intermediate lobe of the pituitary |
| 3. |
Hypothalamus |
| 4. |
Anterior pituitary lobe |
95. In the modern definition, hormones are described as:
| 1. |
Nutrient chemicals that promote digestion. |
| 2. |
Non-nutrient chemicals acting as intercellular messengers. |
| 3. |
Enzymes that accelerate cellular reactions. |
| 4. |
Only chemicals produced by the thyroid gland. |
96. Two flagella are seen in:
| I. |
most dinoflagellates |
| II. |
euglenoids |
1. Only
I
2. Only
II
3. Both
I and
II
4. Neither
I nor
II
97. Adisson’s disease:
| I: |
is caused by excessive secretion of adrenal cortex hormones. |
| II: |
is characterised by altered carbohydrate metabolism causing acute weakness and fatigue. |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
98. Involved in ‘flight or flight response’ which of the following is known to play an important role in stress situations?
1. Thyroxine
2. Insulin
3. Adrenaline
4. Oestrogen
99. Citrus canker is caused by a:
| 1. |
Virus |
2. |
Viroid |
| 3. |
Prion |
4. |
Bacterium |
100. Which dinoflagellate is responsible for causing ‘Red Tide’?
| 1. |
Gonyaulax |
2. |
Noctiluca |
| 3. |
Gymnodinium |
4. |
Ceratium |
101. Select the answer which correctly matches the endocrine gland with the hormone it secretes and its function/deficiency symptom:
|
Endocrine gland |
Hormone |
Function/deficiency symptom |
| (A) |
Posterior Pituitary |
Growth Hormone (GH) |
Oversecretion stimulates abnormal growth |
| (B) |
Thyroid gland |
Thyroxine |
Lack of iodine in diet results in goitre |
| (C) |
Corpus luteum |
Testosterone |
Stimulates spermatogenesis |
| (D) |
Anterior pituitary |
Oxytocin |
Stimulates uterus contraction during childbirth |
1. (A)
2. (B)
3. (C)
4. (D)
102. The primitive prokaryotes responsible for the production of biogas from the dung of ruminant animals, including the:
1. thermoacidophiles
2. methanogens
3. eubacteria
4. halophiles
103. Match the source gland with its respective hormone as well as the function.
|
Source
gland |
Hormone |
Function |
| (a) |
Posterior
pituitary |
Vasopressin |
Stimulates reabsorption
of water in the distal
tubules in the nephron |
| (b) |
Corpus
luteum |
Prolactin |
Supports pregnancy |
| (c) |
Thyroid |
Thyroxine |
Regulates blood calcium
level |
| (d) |
Anterior
pituitary |
Oxytocin |
Contraction of uterus
muscles during childbirth |
1. (a)
2. (b)
3. (c)
4. (d)
104. Graves' disease is characterized by:
1. Underproduction of thyroid hormones
2. Deficiency of vitamin D
3. Hypersensitivity to insulin
4. Overproduction of thyroid hormones
105.
| Assertion (A): |
Diatoms leave behind large amount of cell wall deposits in their habitat, which gets accumulated over billions of years |
| Reason (R): |
-Silicious cell walls in diatoms are indestructible |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
106. Fungi reproduce sexually through a sequence involving:
1. Plasmogamy, karyogamy, and mitosis
2. Plasmogamy, karyogamy, and meiosis
3. Karyogamy, plasmogamy, and budding
4. Spore formation, plasmogamy, and binary fission
107. Methanogens belong to:
1. eubacteria
2. archaebacteria
3. dinoflagellates
4. slime moulds
108. Sleeping sickness in humans is caused by a/an:
1. Amoeboid protozoan
2. Flagellated protozoan
3. Ciliated protozoan
4. Sporozoan
109. Given below are two statements, one is labeled as Assertion (A) and the other is labeled as Reason (R).
| Assertion (A): |
Insulin is a hypoglycaemic hormone. |
| Reason (R): |
Insulin acts mainly on hepatocytes and adipocytes and enhances cellular glucose uptake and utilization. |
In light of the above statements, choose the correct answer from the options given below:
| 1. |
Both (A) and (R) are True and (R) correctly explains (A). |
| 2. |
Both (A) and (R) are True and (R) does not correctly explain (A). |
| 3. |
(A) is True, (R) is False. |
| 4. |
(A) is False, (R) is True |
110. Which of the following statements is correct in relation to the endocrine system?
| 1. |
Organs in the body like the gastrointestinal tract, heart, kidney and liver do not produce any hormones. |
| 2. |
Non-nutrient chemicals produced by the body in trace amounts that act as intercellular messengers are known as hormones. |
| 3. |
Releasing and inhibitory hormones are produced by the pituitary gland. |
| 4. |
Adenohypophysis is under direct neural regulation of the hypothalamus. |
111. Which of the following produces anti-inflammatory reactions and suppresses the immune response?
| 1. |
Thyroxine |
2. |
Thymosin |
| 3. |
Cortisol |
4. |
Aldosterone |
112. Addison’s disease is caused by:
1. Hyperthyroidism
2. Hypothyroidism
3. Hyperadrenalism
4. Hypoadrenalism
113. Which of the following is NOT a feature of viruses?
1. Possessing either DNA or RNA
2. Capable of independent reproduction
3. Being acellular
4. Having a protein coat
114. Consider the given two statements:
| Statement I: |
The classical definition of hormone describes hormone as a chemical produced by exocrine glands, released into the blood and transported to a nearby located target organ. |
| Statement II: |
Current scientific definition describes hormones as non-nutrient chemicals which act as intercellular messengers and are produced in trace amounts. |
1. Statement
I is incorrect; Statement
II is correct
2. Statement
I is correct; Statement
II is incorrect
3. Statement
I is correct; Statement
II is correct
4. Statement
I is incorrect; Statement
II is incorrect
115. The classification of fungi into different classes is primarily based on:
| 1. |
The ecological niche they occupy |
| 2. |
Their mode of nutrient acquisition |
| 3. |
The morphology of the mycelium and reproductive structures |
| 4. |
The presence or absence of symbiotic relationships |
116. Which of the following is not a characteristic of viruses?
1. Obligate parasites
2. Crystalline structure outside host
3. Both RNA and DNA as genetic material
4. Protein coat surrounding nucleic acid
117. What is the main function of melatonin, secreted by the pineal gland?
1. Regulates blood calcium levels
2. Increases heart rate and blood pressure
3. Maintains the sleep-wake cycle and circadian rhythm
4. Stimulates T-lymphocyte maturation
118. Ovary:
| I: |
is the primary female sex organ which normally produces one ovum during each menstrual cycle. |
| II: |
produces two groups of steroid hormones called estrogen and progesterone. |
| III: |
is composed of ovarian follicles and stromal tissues. |
1. Only
I and
II are correct
2. Only
I and
III are correct
3. Only
II and
III are correct
4.
I,
II and
III are correct
119. Which hormone, capable of increasing blood glucose levels, is also a potent anti-inflammatory?
1. Cortisol
2. Thyroxine
3. Melatonin
4. Aldosterone
120. Consider the given two statements:
| Statement I: |
In the five kingdom classification of Whittaker, there is no mention of lichens, viruses, viroids and prions. |
| Statement II: |
Lichens, viruses, viroids and prions are all ‘acellular’ organisms. |
1.
Statement I is correct;
Statement II is correct
2.
Statement I is incorrect;
Statement II is correct
3.
Statement I is correct;
Statement II is incorrect
4.
Statement I is incorrect;
Statement II is incorrect
121. An impairment affecting synthesis or release of ADH results in:
| 1. |
a diminished ability of the kidney to conserve water |
| 2. |
enhanced re-absorption of water by the collecting duct of the nephron |
| 3. |
glycosuria and ketonuria |
| 4. |
a generalised vasoconstriction in the body leading to uncontrolled hypertension |
122. Hormones which interact with membrane-bound receptors:
| I: |
normally do not enter the target cell |
| II: |
generate second messengers which in turn regulate cellular metabolism |
| III: |
include steroid hormones and iodothyronines |
1. Only
I and
II are correct
2. Only
I and
III are correct
3. Only
II and
III are correct
4.
I,
II and
III are correct
123. Which one of the following is wrong for fungi?
| 1. |
They are eukaryotic |
| 2. |
All fungi possess a purely cellulosic cell wall |
| 3. |
They are heterotrophic |
| 4. |
They are both unicellular as well as multicellular |
124. The phrase
‘Contagium vivum fluidum’ is associated with:
| 1. |
M.W. Beijerinck |
2. |
W.M. Stanley |
| 3. |
Louis Pasteur |
4. |
Dmitri Ivanowsky |
125. Consider the given two statements:
| Statement I: |
Fungi are predominantly found in warm and humid places and are cosmopolitan in their distribution, occurring in air, water, soil, and on animals and plants. |
| Statement II: |
Fungi are autotrophic organisms that synthesize their own food from inorganic substances. |
| 1. |
Both Statement I and Statement II are correct and Statement II is the correct explanation for Statement I. |
| 2. |
Both Statement I and Statement II are correct but Statement II is not the correct explanation for Statement I. |
| 3. |
Statement I is correct but Statement II is incorrect. |
| 4. |
Statement I is incorrect but Statement II is correct. |
126. Regarding the given figure:
| I: |
The heterocyst is the location of photosynthetic pigments and oxygen production. |
| II: |
The presence of mucilaginous sheath makes the organism multicellular. |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
127. Match the hormones with their chemical nature:
|
Column I |
|
Column II |
| A. |
Insulin |
P. |
Steroid |
| B. |
Thyroxine |
Q. |
Peptide |
| C. |
Cortisol |
R. |
Amino acid derivative |
Codes:
|
A |
B |
C |
| 1. |
P |
Q |
R |
| 2. |
Q |
R |
P |
| 3. |
R |
P |
Q |
| 4. |
Q |
P |
R |
128. Identify the incorrectly matched pair:
| 1. |
Acromegaly |
Excess of growth hormone in adulthood |
| 2. |
Diabetes insipidus |
Deficiency of insulin |
| 3. |
Graves’ disease |
Hyperthyroidism |
| 4. |
Addison’s disease |
Underproduction of adrenal cortex hormones |
129. The hormone secreted by the alpha cells of the pancreas that raises blood glucose levels by promoting glycogenolysis and gluconeogenesis is:
1. Insulin
2. Glucagon
3. Cortisol
4. Epinephrine
130. The chief ‘producers’ in the oceans are:
1. Cyanobacteria
2. Dinoflagellates
3. Diatoms
4. Lichens
131. Which of the following metabolism is unique to archaebacteria?
| 1. |
Methanogenesis |
2. |
Diazotrophy |
| 3. |
Photosynthesis |
4. |
Methylotrophy |
132. Identify the incorrect statement regarding pancreas:
| 1. |
It is a composite gland which acts as both exocrine and endocrine gland. |
| 2. |
The endocrine pancreas consists of ‘Islets of Langerhans’. |
| 3. |
There are about 1 to 2 million Islets of Langerhans in a normal human pancreas representing 90 percent of the pancreatic tissue. |
| 4. |
It is an organ of the digestive system and endocrine system of vertebrates. |
133. The three-domain system is a biological classification that divides cellular life forms into three domains including all the following except:
1. Archaea
2. Bacteria
3. Eukarya
4. Animalia
134. Identify the incorrect statement regarding viroids:
| 1. |
Viroids are the smallest infectious pathogens known. |
| 2. |
They are composed solely of a short strand of circular, single-stranded RNA that has no protein coating. |
| 3. |
The first recognized viroid, the pathogenic agent of the potato spindle tuber disease, was discovered by Theodor Otto Diener. |
| 4. |
The nucleic acid of viroids codes for only a few proteins. |
135. Which of the following hormones acts on the target cells via a second messenger?
1. Cortisol
2. Adrenocorticotropin [ACTH]
3. Oestrogen
4. Thyroxine
136. Abnormally folded infectious proteins can, in humans, cause:
1. Mad cow disease
2. Scrapie
3. Creutzfeldt-Jacob disease
4. MERS
137. The members of fungal class ‘Deuteromycetes’ are frequently called ‘imperfect fungi’ because:
| 1. |
the sexual cycle has not been observed |
| 2. |
nothing about them indicates their relation to other fungi |
| 3. |
unlike all other fungi, they are unicellular |
| 4. |
they do not reproduce by asexual reproduction |
138. Phylogenetic classification systems are based on:
| 1. |
all observable characteristics. |
| 2. |
cytological information like chromosome number, structure, behaviour. |
| 3. |
evolutionary relationships between the various organisms. |
| 4. |
natural affinities among the organisms and consider, not only the external features, but also internal features, like ultra-structure, anatomy, embryology and phytochemistry. |
139. Identify the incorrect statement:
| 1. |
Hyperadrenalism causes Addison's disease |
| 2. |
A deficiency of insulin causes diabetes mellitus |
| 3. |
Excess of growth hormone in adulthood causes acromegaly |
| 4. |
A severe deficiency of thyroid hormones in childhood leads to cretinism |
140. Pick up the wrong statement.
| 1. |
Cell wall is absent in Animalia. |
| 2. |
Protista have photosynthetic and heterotrophic modes of nutrition |
| 3. |
Some fungi are edible |
| 4. |
Nuclear membrane is present in Monera. |
141. The causative agents of the Potato Spindle Tuber Disease possess:
| 1. |
low molecular weight RNA not enclosed by protein coat |
| 2. |
low molecular weight DNA not enclosed by protein coat |
| 3. |
high molecular weight DNA enclosed by protein coat |
| 4. |
high molecular weight RNA enclosed by protein coat |
142. In Whittaker’s five Kingdom classification, organisms with eukaryotic cell type and cellular body organisation are kept under:
| 1. |
Kingdom Monera |
2. |
Kingdom Protista |
| 3. |
Kingdom Fungi |
4. |
Kingdom Plantae |
143. Consider the given two statements:
| Assertion (A): |
Cortisol increases blood glucose levels. |
| Reason (R): |
Cortisol increases the glucose uptake by the body cells. |
| 1. |
Both (A) and (R) are True and (R) correctly explains (A). |
| 2. |
(A) is True; (R) is False |
| 3. |
(A) is False; (R) is False |
| 4. |
Both (A) and (R) are True but (R) does not correctly explain (A). |
144. Which of the following is not a function of thyroid hormones?
| 1. |
Regulation of metabolic rate |
| 2. |
Promotion of calcium absorption in the intestines |
| 3. |
Stimulation of protein synthesis |
| 4. |
Increase in oxygen consumption and energy expenditure |
145. Name a peptide hormone which acts mainly on hepatocytes, adipocytes and enhances cellular glucose uptake and utilisation:
1. Insulin
2. Glucagon
3. Secretin
4. Gastrin
146. Identify the correct statement:
1. Pineal is stimulated by bright light
2. Pineal secretes melanin
3. Pineal is present in the mediastinum
4. Pineal plays a very important role in diurnal rhythm
147. The pituitary gland
| I: |
is divided anatomically into an adenohypophysis and a neurohypophysis. |
| II: |
Adenohypophysis consists of two portions, pars distalis and pars intermedia. |
| 1. |
Both I and II are correct |
| 2. |
Both I and II are incorrect |
| 3. |
I is correct, II is incorrect |
| 4. |
I is incorrect, II is correct |
148. Consider the two statements:
| Statement I: |
No virus contains both RNA and DNA. |
| Statement II: |
Bacteriophages are usually single-stranded RNA viruses. |
| 1. |
Statement I is incorrect; Statement II is correct |
| 2. |
Statement I is correct; Statement II is incorrect |
| 3. |
Statement I is correct; Statement II is correct |
| 4. |
Statement I is incorrect; Statement II is incorrect |
149. Neurohypophysis:
1. secretes large number of tropins
2. synthesizes oxytocin and vasopressin
3. merges with pars distalis in humans
4. stores and releases oxytocin and vasopressin
150. Identify the incorrect statements regarding phycomycetes:
| I. |
Found in aquatic habitats and on decaying wood in moist and damp places or as obligate parasites on plants. |
| II. |
Mycelium is aseptate and coenocytic. |
| III. |
Asexual reproduction takes place by zoospores (motile) or by aplanospores (non-motile). |
| IV. |
These spores are endogenously produced in sporangium. |
| V. |
A zygospore is formed by the fusion of two gametes. |
1. None, all are correct
2. Only
III
3. Only
II, III, IV and
V
4. Only
I
151. With respect to fungal sexual cycle, choose the correct sequence of events
1. Karyogamy, Plasmogamy and Meiosis
2. Meiosis, Plasmogamy and Karyogamy
3. Plasmogamy, Karyogamy and Meiosis
4. Meiosis, Karyogamy and Plasmogamy
152. Consider the given two statements:
| Assertion (A): |
Deuteromycetes are called "fungi imperfecti" because their sexual reproduction phase is not observed. |
| Reason (R): |
Deuteromycetes reproduce asexually through conidia, and their sexual reproduction structures are not yet discovered. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
153. Select the correct statement.
1. Glucagon is associated with hypoglycemia.
2. Insulin acts on pancreatic cells and adipocytes.
3. Insulin is associated with hyperglycemia.
4. Glucocorticoids stimulate gluconeogenesis.
154. The hypothalamus regulates both the anterior and posterior lobes of the pituitary, but the mechanisms of control differ significantly. Which of the following statements best describes the differences in hypothalamic control over these two lobes?
| 1. |
The hypothalamus controls both lobes through direct neural connections, with specialized neurons releasing regulatory hormones into the bloodstream. |
| 2. |
The anterior pituitary is regulated via a hypothalamo-hypophyseal portal system, while the posterior pituitary is controlled by direct neurosecretory pathways. |
| 3. |
The anterior pituitary releases hormones in response to direct hypothalamic nerve impulses, whereas the posterior pituitary functions independently of hypothalamic signals. |
| 4. |
The anterior pituitary receives hormonal signals through blood circulation, while the posterior pituitary releases hormones independently without hypothalamic influence. |
155. The hormone that triggers the release of glucose from glycogen stores in the liver is:
| 1. |
Insulin |
2. |
Glucagon |
| 3. |
Cortisol |
4. |
Thyroxine |
156. Nostoc:
| I: |
is a cyanobacterium. |
| II: |
is photosynthetic autotroph. |
| III: |
is capable of fixing atmospheric nitrogen. |
1. Only
I and
II are correct
2. Only
I and
III are correct
3. Only
II and
III are correct
4.
I,
II and
III are correct
157. Consider the following two statements:
Nitrogen-fixing organisms belong to
I: Kingdom Monera
II: Kingdom Protista
| 1. |
Only I is correct |
| 2. |
Only II is correct |
| 3. |
Both I and II are correct |
| 4. |
Neither I nor II are correct |
158. The thyroid gland:
| I: |
is composed of two lobes interconnected with a thin flap of connective tissue called isthmus. |
| II: |
is composed of follicles and stromal tissues. |
| III: |
is located in the mediastinum, ventral to aorta. |
1. Only
I and
II are correct
2. Only
I and
III are correct
3. Only
II and
III are correct
4.
I,
II and
III are correct
159. The deficiency of anti-diuretic hormone (ADH) can lead to:
| 1. |
Diabetes mellitus |
2. |
Diabetes insipidus |
| 3. |
Pituitary diabetes |
4. |
Adrenal diabetes |
160. The Mycoplasma:
| I: |
are organisms that completely lack a cell wall. |
| II: |
are the smallest living cells known and can survive without oxygen. |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
161. The rod-shaped bacteria belongs to which of the following category of bacteria?
1. Vibrio
2. Bacillus
3. Coccus
4. Spirillum
162. Nostoc:
| 1. |
is a green alga |
| 2. |
is unicellular eukaryote capable of forming filamentous colonies |
| 3. |
can fix atmospheric nitrogen |
| 4. |
carry out anoxygenic photosynthesis |
163. All the following regarding insulin are correct except:
| 1. |
Insulin lowers blood glucose levels by promoting glucose uptake into cells. |
| 2. |
It promotes glycogenesis in liver and muscle cells. |
| 3. |
Insulin inhibits gluconeogenesis in the liver. |
| 4. |
Insulin increases the breakdown of fat in adipose tissue. |
164. Addison’s disease is:
| 1. |
hypothyroidism |
2. |
hyperthyroidism |
| 3. |
hypoadrenalism |
4. |
hypopituitarism |
165. Which one of the following statements is wrong?
| 1. |
Golden algae are also called desmids |
| 2. |
Eubacteria are also called false bacteria. |
| 3. |
Phycomycetes are also called algal fungi. |
| 4. |
Cyanobacteria are also called blue-green algae. |
166. A protein hormone that regulates the blood calcium levels is secreted by:
a. Thyroid
b. Parathyroid
1. Only a
2. Only b
3. Both a and b
4. Neither a nor b
167. Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
| Assertion (A): |
FSH which interacts with membrane bound receptors does not enter the target cell. |
| Reason (R): |
Binding of FSH to its receptors generates second messenger (cyclic AMP) for its biochemical and physiological responses. |
In the light of the above statements, choose the most appropriate answer from the options given below:
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
168. A steroid hormone when released increases blood glucose level, causes lipolysis and proteolysis, retards cellular uptake and utilization of amino acids by body cells and has a potent anti-inflammatory effect. This hormone is:
1. Thyroxin
2. Adrenaline
3. Growth hormone
4. Cortisol
169. Match the following organisms with their respective groups in Protista:
|
Column I |
|
Column II |
| A. |
Diatoms |
P. |
Protozoa |
| B. |
Paramecium |
Q. |
Chrysophytes |
| C. |
Slime molds |
R. |
Fungi-like protists |
| D. |
Kelps |
S. |
Not listed in Protista |
Codes:
|
A |
B |
C |
D |
| 1. |
Q |
P |
R |
S |
| 2. |
P |
Q |
R |
S |
| 3. |
Q |
P |
S |
R |
| 4. |
S |
P |
Q |
R |
170. Identify the correct statements:
| I: |
Binding of a hormone to its receptor leads to the formation of a hormone-receptor complex. |
| II: |
Each receptor is specific to one hormone only and hence receptors are specific. |
| III: |
Hormone-Receptor complex formation leads to certain biochemical changes in the target tissue. |
1. Only
I and
II
2. Only
I and
III
3. Only
II and
III
4.
I,
II and
III
171. With regard to hormones, identify the correct statements.
| A. |
Epinephrine is a peptide hormone. |
| B. |
Progesterone is a peptide hormone. |
| C. |
Hormones that interact with membrane bound receptors normally do not enter the target cell, but generate second messengers. |
| D. |
Hormones that interact with intracellular receptors mostly regulate gene expression. |
| E. |
Insulin is an amino acid derivative hormone. |
Choose the most appropriate answer from the options given below:
| 1. |
A and B only |
2. |
C and E only |
| 3. |
C and D only |
4. |
B and C only |
172. Consider the given two statements:
| Statement I: |
Archaebacteria can survive in extreme conditions. |
| Statement II: |
Methanogens are archaebacteria. |
| 1. |
Statement I is correct; Statement II is correct |
| 2. |
Statement I is incorrect; Statement II is correct |
| 3. |
Statement I is correct; Statement II is incorrect |
| 4. |
Statement I is incorrect; Statement II is incorrect |
173. In 1969, who proposed the five kingdom classification for living organisms?
| 1. |
R.H. Whittaker |
2. |
C.Linnaeus |
| 3. |
A. Roxberg |
4. |
Virchow |
174. Which of the following are most suitable indicators of SO2 pollution in the environment?
1. Lichens
2. Conifers
3. Algae
4. Fungi
175. What would be true for
Alternaria but not for
Puccinia?
| 1. |
Heterotrophic mode of nutrition |
| 2. |
Sexual stage is unknown |
| 3. |
Lack of vegetative reproduction |
| 4. |
Called as bracket fungi |
176. Consider the given two statements:
| Statement I: |
A hypothalamic hormone called Gonadotrophin releasing hormone (GnRH) stimulates the synthesis and release of gonadotrophins by gonads. |
| Statement II: |
Somatostatin from the hypothalamus stimulates the release of growth hormone from the pituitary. |
1.
Statement I is incorrect;
Statement II is correct
2.
Statement I is correct;
Statement II is incorrect
3.
Statement I is correct;
Statement II is correct
4.
Statement I is incorrect;
Statement II is incorrect
177. Consider the two given statements:
| Statement I: |
Association between mycobiont and phycobiont is found in Lichens. |
| Statement II: |
An association between roots of higher plants and fungi is called mycorrhiza. |
1.
Statement I is correct;
Statement II is incorrect
2.
Statement I is correct;
Statement II is correct
3.
Statement I is incorrect;
Statement II is incorrect
4.
Statement I is incorrect;
Statement II is correct
178. Identify the correct statements regarding Dinoflagellates:
| I: |
They appear yellow, green, brown, blue or red, depending on the main pigments present in their cells |
| II: |
The cell wall has stiff cellulosic plates on the outer surface |
| III: |
Most of them have single flagellum |
1. Only
I and
II
2. Only
I and
III
3. Only
II and
III
4.
I,
II and
III
179. The vast majority of bacteria:
| 1. |
are capable of fixing atmospheric nitrogen. |
| 2. |
are photosynthetic autotrophs and have gas vacuoles. |
| 3. |
do not have peptidoglycan in their cell walls. |
| 4. |
depend on other organisms or on dead organic matter for food. |
180. The thymus gland is a lobular structure located:
| 1. |
between lungs behind sternum on the dorsal side of aorta. |
| 2. |
between lungs behind sternum on the ventral side of aorta. |
| 3. |
on the dorsal side of the forebrain. |
| 4. |
on the ventral side of the forebrain. |
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course
