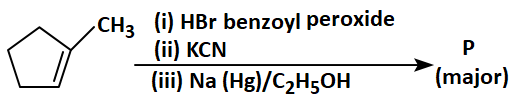PHYSICS
1. A parallel plate capacitor made of circular plates is being charged such that the surface charge density on its plates is increasing at a constant rate with time. The magnetic field arising due to displacement current is:
| 1. |
non-zero everywhere with a maximum at the imaginary cylindrical surface connecting the peripheries of the plates. |
| 2. |
zero between the plates and non-zero outside. |
| 3. |
zero at all places. |
| 4. |
constant between the plates and zero outside the plates. |
2. An electric dipole with dipole moment
\(5\times10^{-6}~\text{C-m} \) is aligned with the direction of a uniform electric field of magnitude
\(4\times10^{5}~\text{N/C}. \) The dipole is then rotated through an angle of
\(60^\circ\) with respect to the electric field. The change in the potential energy of the dipole is:
| 1. |
\(1.2~\text{J}\) |
2. |
\(1.5~\text{J}\) |
| 3. |
\(0.8~\text{J}\) |
4. |
\(1.0~\text{J}\) |
3. A ball of mass \(0.5~\text{kg}\) is dropped from a height of \(40~\text{m}.\) The ball hits the ground and rises to a height of \(10~\text{m}.\) The impulse imparted to the ball during its collision with the ground is:
(take \(g=9.8~\text{m/s}^2\))
1. \(0\)
2. \(84~\text{N-s}\)
3. \(21~\text{N-s}\)
4. \(7~\text{N-s}\)
4. The intensity of transmitted light when a polaroid sheet, placed between two crossed polaroids at
\(22.5^\circ\) from the polarization axis of one of the polaroids, is (
\(I_0\) is the intensity of polarised light after passing through the first polaroid):
| 1. |
\(\dfrac{I_0}{8}\) |
2. |
\(\dfrac{I_0}{16}\) |
| 3. |
\(\dfrac{I_0}{2}\) |
4. |
\(\dfrac{I_0}{4}\) |
5. The kinetic energies of two similar cars
\(A\) and
\(B\) are
\(100~\text J\) and
\(225~\text J\) respectively. On applying brakes, car
\(A\) stops after
\(1000~\text m\) and car
\(B\) stops after
\(1500~\text m.\) If
\(F_A\) and
\(F_B\) are the forces applied by the brakes on cars
\(A\) and
\(B,\) respectively, then the ratio
\(\dfrac{F_A}{F_B}\) is:
| 1. |
\(\dfrac{1}{3}\) |
2. |
\(\dfrac{1}{2}\) |
| 3. |
\(\dfrac{3}{2}\) |
4. |
\(\dfrac{2}{3}\) |
6. The current passing through the battery in the given circuit, is:

| 1. |
\(2.5~\text{A}\) |
2. |
\(1.5~\text{A}\) |
| 3. |
\(2.0~\text{A}\) |
4. |
\(0.5~\text{A}\) |
7. Three identical heat conducting rods are connected in series as shown in the figure. The rods on the sides have thermal conductivity
\(2K\) while that in the middle has thermal conductivity
\(K\). The left end of the combination is maintained at temperature
\(3T\) and the right end at
\(T.\) The rods are thermally insulated from outside. In the steady state, temperature at the left junction is
\(T_1\) and that at the right junction is
\(T_2\). The ratio
\(\dfrac{T_1}{T_2}\) is:

| 1. |
\(\dfrac{5}{3}\) |
2. |
\(\dfrac{5}{4}\) |
| 3. |
\(\dfrac{3}{2}\) |
4. |
\(\dfrac{4}{3}\) |
8. In an oscillating spring mass system, a spring is connected to a box filled with the sand. As the box oscillates, sand leaks slowly out of the box vertically so that the average frequency
\(\omega~(t)\) and average amplitude
\(A~(t)\) of the system changes with time
\(t.\) Which of the following options systemically depicts these changes correctly?
9. \(AB\) is a part of an electrical circuit (see figure). The potential difference
\(''V_{A}-V_{B}'',\) at the instant when current
\(i=2~\text A\) and is increasing at a rate of
\(1~\text{amp/second}\) is:

| 1. |
\(9~ \text{volts}\) |
2. |
\(10~ \text{volts}\) |
| 3. |
\(5~ \text{volts}\) |
4. |
\(6~ \text{volts}\) |
10. A particle of mass
\(m\) is moving around the origin with a constant force
\(F\) pulling it towards the origin. If Bohr model is used to describe its motion, the radius
\(r\) of the
\(n^{\text{th}}\) orbit and the particle's speed
\(v\) in the orbit depend on
\(n\) as:
| 1. |
\(r \propto n^{2/3}; v \propto n^{1/3}\) |
| 2. |
\(r \propto n^{4/3}; v \propto n^{-1/3}\) |
| 3. |
\(r \propto n^{1/3}; v \propto n^{1/3}\) |
| 4. |
\(r \propto n^{1/3}; v \propto n^{2/3}\) |
11. In some appropriate units, time
\((t)\) and position
\((x)\) relation of a moving particle is given by
\(t=x^2+x. \) The acceleration of the particle is:
| 1. |
\(+\dfrac{2}{(x+1)^3}\) |
2. |
\(+\dfrac{2}{(2x+1)}\) |
| 3. |
\(-\dfrac{2}{(x+2)^3}\) |
4. |
\(-\dfrac{2}{(2x+1)^3}\) |
12. A model for the quantized motion of an electron in a uniform magnetic field
\(B\) states that the flux passing through the orbit of the electron is
\(n(h/e)\) where
\(n\) is an integer,
\(h\) is Planck's constant and
\(e\) is the magnitude of the electron's charge. According to the model, the magnetic moment of an electron is its lowest energy state will be (
\(m\) is the mass of the electron):
| 1. |
\(\dfrac{heB}{\pi m}\) |
2. |
\(\dfrac{heB}{2\pi m}\) |
| 3. |
\(\dfrac{he}{\pi m}\) |
4. |
\(\dfrac{he}{2\pi m}\) |
13. A microscope has an objective of focal length \(2~\text{cm},\) eyepiece of focal length \(4~\text{cm}\) and the tube length of \(40~\text{cm}.\) If the distance of distinct vision of eye is \(25~\text{cm},\) the magnification in the microscope is:
1. \(150\)
2. \(250\)
3. \(100\)
4. \(125\)
14. There are two inclined surfaces of equal length
\((L)\) and same angle of inclination
\(45^\circ\) with the horizontal. One of them is rough and the other is perfectly smooth. A given body takes
\(2\) times as much time to slide down on rough surface than on the smooth surface. The coefficient of kinetic friction
\((\mu_k)\) between the object and the rough surface is close to:
| 1. |
\(0.5\) |
2. |
\(0.75\) |
| 3. |
\(0.25\) |
4. |
\(0.40\) |
15. A full wave rectifier circuit with diodes
\((D_1)\) and
\((D_2)\) is shown in the figure. If input supply voltage
\(V_{\text{in}}=220\sin(100\pi t)\) volt, then at
\(t=15~\text{ms}\text{:}\)

| 1. |
\(D_1\) and \(D_2\) both are forward biased |
| 2. |
\(D_1\) and \(D_2\) both are reverse biased |
| 3. |
Neither \(D_1\) nor \(D_2\) conducts at any time |
| 4. |
\(D_1\) is reverse biased, \(D_2\) is forward biased |
16. A uniform rod of mass
\(20~\text{kg}\) and length
\(5\text{ m}\) leans against a smooth vertical wall making an angle of
\(60^{\circ}\) with it. The other end rests on a rough horizontal floor. The friction force that the floor exerts on the rod is: (take
\(g=10~\text{m/s}^2\) )
| 1. |
\(200 ~\text{N}\) |
2. |
\(200 \sqrt{3} ~\text{N}\) |
| 3. |
\(100 ~\text{N}\) |
4. |
\(100 \sqrt{3} ~\text{N}\) |
17. Two identical charged conducting spheres \(A\) and \(B\) have their centres separated by a certain distance. Charge on each sphere is \(q\) and the force of repulsion between them is \(F. \) A third identical uncharged conducting sphere is brought in contact with sphere \(A\) first and then with \(B \) and finally removed from both. New force of repulsion between spheres \( A\) and \(B \) (Radii of \(A\) and \(B \) are negligible compared to the distance of separation so that for calculating force between them they can be considered as point charges) is best given as:
1. \(\dfrac{F}{2}\)
2. \(\dfrac{3 F}{8}\)
3. \(\dfrac{3 F}{5}\)
4. \(\dfrac{2 F}{3}\)
18. Two cities \(X \) and \(Y\) are connected by a regular bus service with a bus leaving in either direction every \(T~\text{min}.\) A girl is driving scooty with a speed of \(60~\text{km/h}\) in the direction \(X\) to \(Y\) notices that a bus goes past her every \(30~\text{minutes}\) in the direction of her motion, and every \(10~\text{minutes}\) in the opposite direction. Choose the correct option for the period \(T\) of the bus service and the speed (assumed constant) of the buses.
1. \(10 ~\text{min},~ 90~ \text{km/h}\)
2. \(15 ~\text{min},~ 120~ \text{km/h}\)
3. \(9 ~\text{min},~ 40~ \text{km/h}\)
3. \(25 ~\text{min},~ 100~ \text{km/h}\)
19. A container has two chambers of volumes \(V_1=2~\text{litres} \) and \(V_2=3~\text{litres} \) separated by a partition made of a thermal insulator. The chambers contains \( n_1=5 \) and \( n_2=4 \) moles of ideal gas at pressures \(p_1=1~\text{atm} \) and \(p_2=2~\text{atm}, \) respectively. When the partition is removed, the mixture attains an equilibrium pressure of:
1. \(1.4 ~\text{atm} \)
2. \(1.8 ~\text{atm} \)
3. \(1.3 ~\text{atm} \)
4. \(1.6 ~\text{atm} \)
20. De-Broglie wavelength of an electron orbiting in the \(n=2\) state of hydrogen atom is close to: (Given: Bohr radius \(=0.052~ \text{nm}\))
1. \(1.67~ \text{nm}\)
2. \(2.67~ \text{nm}\)
3. \(0.067~ \text{nm}\)
4. \(0.67~ \text{nm}\)
21. To an AC power supply of
\(220~\text V\) at
\(50~\text{Hz},\) a resistor of
\(20~\Omega,\) a capacitor of reactance
\(25~\Omega\) and an inductor of reactance
\(45~\Omega\) are connected in series. The corresponding current in the circuit and the phase angle between the current and the voltage is, respectively:
| 1. |
\(15.6~\text {A and} ~30^\circ\) |
2. |
\(15.6~\text {A and} ~45^\circ\) |
| 3. |
\(7.8~\text {A and} ~30^\circ\) |
4. |
\(7.8~\text {A and} ~45^\circ\) |
22. Which of the following options represents the variation of photoelectric current with the property of light shown on the
\(x \text{-}\)axis?
| \(\mathrm{(A)}\) |
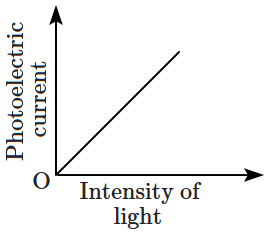 |
\(\mathrm{(B)}\) |
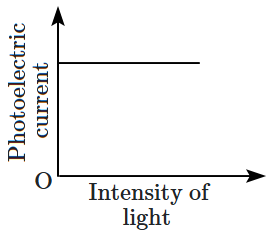 |
| \(\mathrm{(C)}\) |
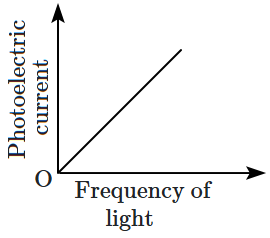 |
\(\mathrm{(D)}\) |
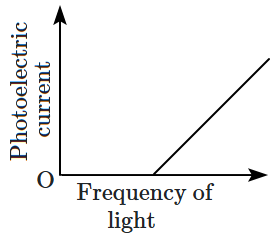 |
1.
\(\mathrm{(A)}\) and
\(\mathrm{(D)}\)
2.
\(\mathrm{(B)}\) and
\(\mathrm{(D)}\)
3.
\(\mathrm{(A)}\) only
4.
\(\mathrm{(A)}\) and
\(\mathrm{(C)}\)
23. A pipe open at both ends has a fundamental frequency
\(f\) in air. The pipe is now dipped vertically in a water drum to half of its length. The fundamental of the air column is now equal to:
| 1. |
\(\dfrac{3f}{2}\) |
2. |
\(2f\) |
| 3. |
\(\dfrac{f}{2}\) |
4. |
\(f\) |
24. Two identical point masses
\(P\) and
\(Q,\) suspended from two separate massless springs of spring constant
\(k_1\) and
\(k_2,\) respectively, oscillate vertically. If their maximum speeds are the same, the ratio
\(\left(\dfrac{A_Q}{A_P} \right)\) of the amplitude
\(A_Q\) of mass
\(Q \) to the amplitude
\(A_P\) of mass
\(P\) is:
| 1. |
\(\sqrt{\dfrac{k_2}{k_1}}\) |
2. |
\(\sqrt{\dfrac{k_1}{k_2}}\) |
| 3. |
\(\dfrac{k_2}{k_1}\) |
4. |
\(\dfrac{k_1}{k_2}\) |
25. The output
\(Y\) of the given logic implementation is similar to the output of which gate?

1. OR
2. NOR
3. AND
4. NAND
26. An oxygen cylinder of volume
\(30\) litre has
\(18.20\) moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to
\(11\) atmospheric pressure at temperature
\(27^{\circ} \text{C}.\) The mass of the oxygen withdrawn from the cylinder is nearly equal to:
\([\)Given,
\(R=\frac{100}{12}~ \text{J} \mathrm{~mol}^{-1} {~\text K}^{-1},\) and molecular mass of
\(O_2=32,\) \(1\) atm pressure
\(\left.=1.01 \times 10^5 \mathrm{~N} / \mathrm{m}\right]\)
| 1. |
\(0.116\text{ kg}\) |
2. |
\(0.156\text{ kg}\) |
| 3. |
\(0.125\text{ kg}\) |
4. |
\(0.144\text{ kg}\) |
27. In a certain camera, a combination of four similar thin convex lenses are arranged axially in contact. Then the power of the combination and the total magnification in comparison to the power \(( p )\) and magnification \(( m )\) for each lens will be, respectively -
1. \(4 p\) and \(m^4\)
2. \(p^4\) and \(m^4\)
3. \(4 p\) and \(4 m\)
4. \(p^4\) and \(4 m\)
28. Two gases
\(A\) and
\(B\) are filled at the same pressure in separate cylinders with movable pistons of radius
\(r_A\) and
\(r_B,\) respectively. On supplying an equal amount of heat to both the systems reversibly under constant pressure, the pistons of gas
\(A\) and
\(B\) are displaced by
\(16~ \text{cm}\) and
\(9~ \text{cm},\) respectively. If the change in their internal energy is the same, then the ratio
\(r_A / r_B\) is equal to:
| 1. |
\(\dfrac{2}{\sqrt{3}}\) |
2. |
\(\dfrac{\sqrt{3}}{2}\) |
| 3. |
\(\dfrac{4}{3}\) |
4. |
\(\dfrac{3}{4}\) |
29. A balloon is made of a material of surface tension
\(S\) and its inflation outlet (from where gas is filled in it) has small area
\(A.\) It is filled with a gas of density
\(\rho\) and takes a spherical shape of radius
\(R.\) When the gas is allowed to flow freely out of it, its radius
\(r\) changes from
\(R\) to
\(0\) (zero) in time
\(T.\) If the speed
\(v(r)\) of gas coming out of the balloon depends on
\(r\) as
\(r^a\) and
\(T \propto S^\alpha A^\beta \rho^\gamma R^\delta\) then:
| 1. |
\(a=-\dfrac{1}{2},~ \alpha=-\dfrac{1}{2}, ~\beta=-1, ~\gamma=\dfrac{1}{2},~ \delta=\dfrac{7}{2}\) |
| 2. |
\(a=\dfrac{1}{2},~\alpha=\dfrac{1}{2},~ \beta=-\dfrac{1}{2}, ~\gamma=\dfrac{1}{2},~ \delta=\dfrac{7}{2}\) |
| 3. |
\(a=\dfrac{1}{2}, ~\alpha=\dfrac{1}{2}, ~\beta=-1, ~\gamma=+1, ~\delta=\dfrac{3}{2}\) |
| 4. |
\(a=-\dfrac{1}{2}, ~\alpha=-\dfrac{1}{2}, ~\beta=-1, ~\gamma=-\dfrac{1}{2}, ~\delta=\dfrac{5}{2}\) |
30. A bob of heavy mass
\(m\) is suspended by a light string of length
\(l.\) The bob is given a horizontal velocity
\(v_0\) as shown in figure. If the string gets slack at some point
\(P\) making an angle
\(\theta \) from the horizontal, the ratio of the speed
\(v\) of the bob at point
\(P\) to its initial speed
\(v_0\) is:

| 1. |
\(\left(\dfrac{\cos \theta}{2+3 \sin \theta}\right)^{-1 / 2}\) |
2. |
\(\left(\dfrac{\sin \theta}{2+3 \sin \theta}\right)^{1 / 2}\) |
| 3. |
\((\sin \theta)^{1 / 2}\) |
4. |
\(\left(\dfrac{1}{2+3 \sin \theta}\right)^{1 / 2}\) |
31. A physical quantity \(P\) is related to four observations \(a,b,c\) and \(d\) as follows:
\(P=\dfrac{a^3b^2}{c\sqrt{d}}\)
The percentage errors of measurement in \(a,b,c\) and \(d\) are \(1\%,3\%,2\%\) and \(4\%\) respectively. The percentage error in the quantity \(P\) is:
1. \(13\%\)
2. \(15\%\)
3. \(10\%\)
4. \(2\%\)
32. The sun rotates around its centre once in
\(27\) days. What will be the period of revolution if the sun were to expand to twice its present radius without any external influence? Assume the sun to be a sphere of uniform density.
| 1. |
\(115\) days |
2. |
\(108\) days |
| 3. |
\(100\) days |
4. |
\(105\) days |
33. The radius of Martian orbit around the sun is about \(4\) times the radius of the orbit of mercury. The Martian year is \(687\) earth days. Then which of the following is the length of \(1\) year on mercury?
1. \(172\) earth days
2. \(124\) earth days
3. \(88\) earth days
4. \(225\) earth days
34. A wire of resistance
\(R\) is cut into
\(8\) equal pieces. From these pieces two equivalent resistance are made by adding four of these together in parallel. Then these two sets are added in series. The net effective resistance of the combination is:
| 1. |
\(\dfrac{R}{16}\) |
2. |
\(\dfrac{R}{8}\) |
| 3. |
\(\dfrac{R}{64}\) |
4. |
\(\dfrac{R}{32}\) |
35. A photon and an electron (mass
\(m\)) have the same energy
\(E.\) The ratio
\((\lambda_{\text{photon}}/\lambda_{\text{electron}})\) of their de Broglie wavelengths is: (
\(c\) is the speed of light)
| 1. |
\(c\sqrt{\dfrac{2m}{E}}\) |
2. |
\(\dfrac{1}{c}\sqrt{\dfrac{E}{2m}}\) |
| 3. |
\(\sqrt{\dfrac{E}{2m}}\) |
4. |
\(c\sqrt{2mE}\) |
36. A sphere of radius
\(R\) is cut from a larger solid sphere of radius
\(2R\) as shown in the figure. The ratio of the moment of inertia of the smaller sphere to that of the rest part of the sphere about the
\(Y\)-axis is:

| 1. |
\(\dfrac{7}{57}\) |
2. |
\(\dfrac{7}{64}\) |
| 3. |
\(\dfrac{7}{8}\) |
4. |
\(\dfrac{7}{40}\) |
37. An electron (mass
\(9\times10^{-31}~\text{kg}\) and charge
\(1.6\times10^{-19}~\text C\)) moving with speed
\(c/100\) (
\(c\)=speed of light) is injected into a magnetic field
\(\vec B\) of magnitude
\(9\times10^{-4}~\text{T}\) perpendicular to its direction of motion. We wish to apply an uniform electric field
\(\vec E\) together with the magnetic field so that the electron does not deflect from its path. Then (speed of light
\(c=3\times10^8~\text{ms}^{-1}\))
| 1. |
\(\vec E\) is parallel to \(\vec B\) and its magnitude is \(27\times10^{2}~\text{V m}^{-1}\) |
| 2. |
\(\vec E\) is parallel to \(\vec B\) and its magnitude is \(27\times10^{4}~\text{V m}^{-1}\) |
| 3. |
\(\vec E\) is perpendicular to \(\vec B\) and its magnitude is \(27\times10^{4}~\text{V m}^{-1}\) |
| 4. |
\(\vec E\) is perpendicular to \(\vec B\) and its magnitude is \(27\times10^{2}~\text{V m}^{-1}\) |
38. The electric field in a plane electromagnetic wave is given by
\(E_z=60\cos(5x+1.5\times10^9t)~\text{V/m}.\) Then expression for the corresponding magnetic field is (here subscripts denote the direction of the field):
| 1. |
\(B_z=60\cos(5x+1.5\times10^9t)~\text T\) |
| 2. |
\(B_y=60\sin(5x+1.5\times10^9t)~\text T\) |
| 3. |
\(B_y=2\times10^{-7}\cos(5x+1.5\times10^9t)~\text T\) |
| 4. |
\(B_x=2\times10^{-7}\cos(5x+1.5\times10^9t)~\text T\) |
39. A body weight \(48~\text{N}\) on the surface of the earth. The gravitational force experienced by the body due to the Earth at a height equal to one-third the radius of the Earth from its surface is:
1. \(32~\text N\)
2. \(36~\text N\)
3. \(16~\text N\)
4. \(27~\text N\)
40. An unpolarized light beam travelling in air is incident on a medium of refractive index
\(1.73\) at Brewster's angle. Then:
| 1. |
both reflected and transmitted light are perfectly polarized with angles of reflection and refraction close to \(60^\circ\) and \(30^\circ,\) respectively. |
| 2. |
transmitted light is completely polarized with angle of refraction close to \(30^\circ\) |
| 3. |
reflected light is completely polarized and the angle of reflection is close to \(60^\circ\) |
| 4. |
reflected light is partially polarized and the angle of reflection is close to \(30^\circ\) |
41. A constant voltage of
\(50~\text{V}\) is maintained between the points
\(A\) and
\(B\) of the circuit shown in the figure. The current through the branch
\(C D\) of the circuit is:

| 1. |
\(2.5~A\) |
2. |
\(3.0~A\) |
| 3. |
\(1.5~A\) |
4. |
\(2.0~A\) |
42. The plates of a parallel plate capacitor are separated by
\(d.\) Two slabs of different dielectric constant
\(K_1\) and
\(K_2\) with thickness
\(\dfrac{3}{8} d\) and
\(\dfrac{d}{2},\) respectively are inserted in the capacitor. Due to this, the capacitance become two times larger than when there is nothing between the plates.
If
\(K_1=1.25 ~K_2,\) the value of
\(K_1\) is:
| 1. |
\(1.60\) |
2. |
\(1.33\) |
| 3. |
\(2.66\) |
4. |
\(2.33\) |
43. Consider the diameter of a spherical object being measured with the help of a Vernier callipers. Suppose its \(10\) Vernier Scale Divisions (V.S.D.) are equal to its \(9\) Main Scale Divisions (M.S.D.). The least division in the M.S. is \(0.1\text{ cm}\) and the zero of V.S. is at \(x=0.1~ \text{cm}\) when the jaws of Vernier callipers are closed. If the main scale reading for the diameter is \({M}=5~\text{cm}\) and the number of coinciding vernier division is \(8 ,\) the measured diameter after zero error correction, is:
1. \(4.98 ~\text{cm}\)
2. \(5.00 ~\text{cm}\)
3. \(5.18 ~\text{cm}\)
4. \(5.08 ~\text{cm}\)
44. A \(2~\text{amp}\) current is flowing through two different small circular copper coils having radii ratio \(1:2.\) The ratio of their respective magnetic moments will be:
1. \(2: 1\)
2. \(4: 1\)
3. \(1: 4\)
4. \(1: 2\)
45. Consider a water tank shown in the figure. It has one wall at
\(x=L\) and can be taken to be very wide in the
\(z\) direction. When filled with a liquid of surface tension
\(S\) and density
\(\rho,\) the liquid, surface makes angle
\(\theta_{0}\left(\theta_0 \ll 1\right)\) with the
\(x\text-\)axis at
\(x=L.\) If
\(y(x)\) is the height of the surface then the equation for
\(y(x)\) is:

(take
\(\theta(x)=\sin \theta(x)=\tan \theta(x)=\dfrac{d y}{d x}, g\) is the acceleration due to gravity)
| 1. |
\(\dfrac{d^2 y}{d x^2}=\sqrt{\dfrac{\rho g}{S}}\) |
2. |
\(\dfrac{d y}{d x}=\sqrt{\dfrac{\rho g}{S}} x\) |
| 3. |
\(\dfrac{d^2 y}{d x^2}=\dfrac{\rho g}{S} x\) |
4. |
\(\dfrac{d^2 y}{d x^2}=\dfrac{\rho g}{S} y\) |
CHEMISTRY
46. Identify the suitable reagent for the following conversion:

1. (i)
\(NaBH_4\), (ii)
\(H^+/H_2O\)
2.
\(H_2/ Pd-BaSO_4\)
3. (i)
\(LiAlH_4\), (ii)
\(H^+/H_2O\)
4. (i)
\(AlH(iBu)_2\)(ii)
\(H_2O\)
47. The correct order of decreasing acidity of the following aliphatic acids is:
| 1. |
\(\small {\mathrm{HCOOH}>\mathrm{CH}_3 \mathrm{COOH}> \left(\mathrm{CH}_3\right)_2 \mathrm{CHCOOH} }\)
\(\small {> \left(\mathrm{CH}_3\right)_3 \mathrm{CCOOH}} \) |
| 2. |
\(\small {\mathrm{HCOOH}>\left(\mathrm{CH}_3\right)_3 \mathrm{CCOOH}> \left(\mathrm{CH}_3\right)_2 \mathrm{CHCOOH}}\)
\( \small{ > \mathrm{CH}_3 \mathrm{COOH}} \) |
| 3. |
\(\small { \left(\mathrm{CH}_3\right)_3 \mathrm{CCOOH}>\left(\mathrm{CH}_3\right)_2 \mathrm{CHCOOH}> \mathrm{CH}_3 \mathrm{COOH}}\)
\(\small {>\mathrm{HCOOH}} \) |
| 4. |
\(\small {\mathrm{CH}_3 \mathrm{COOH}>\left(\mathrm{CH}_3\right)_2 \mathrm{CHCOOH}> \left(\mathrm{CH}_3\right)_3\mathrm{CCOOH} }\)
\(\small {>\mathrm{HCOOH}} \) |
48. Which one of the following reactions does not belong to "Lassaigne's test"?
| 1. |
\(\mathrm{Na}+\mathrm{X} \underset{\Delta}{\longrightarrow}+\mathrm{NaX} \) |
| 2. |
\(2 \mathrm{CuO}+\mathrm{C} \xrightarrow[\Delta]{} 2 \mathrm{Cu}+\mathrm{CO}_2 \) |
| 3. |
\(\mathrm{Na}+\mathrm{C}+\mathrm{N} \xrightarrow[\Delta]{} \mathrm{NaCN} \) |
| 4. |
\(2 \mathrm{Na}+\mathrm{S} \xrightarrow[\Delta]{} \mathrm{Na}_2 \mathrm{~S}\) |
49. If the rate constant of a reaction is
\(0.03 s^{-1}\), how much time does it take for
\(7.2\text { mol L}^{-1}\) concentration of the reactant to get reduced to
\(0.9~\text {mol L} ^{-1}?\)
(Given: log 2=0.301)
| 1. |
210 s |
2. |
21.0 s |
| 3. |
69.3 s |
4. |
23.1 s |
50. Given below are two statements:
| Statement I: |
A hypothetical diatomic molecule with bond order zero is quite stable. |
| Statement II: |
As bond order increases, the bond length increases. |
| 1. |
Statement I is True but Statement II is False. |
| 2. |
Statement I is False but Statement II is True. |
| 3. |
Both Statement I and Statement II are True. |
| 4. |
Both Statement I and Statement II are False. |
51. Out of the following complex compounds, which of the compounds will be having the minimum conductance in solution?
| 1. |
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3 \) |
2. |
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{Cl} \) |
| 3. |
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right] \) |
4. |
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right]\) |
52. Which of the following aqueous solution will exhibit highest boiling point?
1. \(0.01 \mathrm{M} ~\mathrm{Na}_2 \mathrm{SO}_4 \)
2. \(0.015 \text M~\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6 \)
3. \(0.01 \text {M Urea} \)
4. \(0.01 \mathrm{M} ~\mathrm{KNO}_3\)
53. Given below are two statements : one is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
 undergoes \(S_N2\) reaction faster than undergoes \(S_N2\) reaction faster than  |
| Reason (R): |
Iodine is a better leaving group because of its larger size. |
In the light of the above statements, choose the correct answer from the options given below :
| 1. |
(A) is True but (R) is False |
| 2. |
(A) is False but (R) is True |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A) |
| 4. |
Both (A) and (R) are True but (R) is not the correct explanation of (A) |
54. Consider the following compounds:
\(\underline KO_2, H_2 \underline O_2\) and \(H_2 \underline SO_4\)
The oxidation states of the underlined elements in them are, respectively:
1. +1, -2 and +4
2. +4 , -4 and +6
3. +1, -1 and +6
4. +2, -2 and +6
55. Match
List-I with
List-II
|
List-I |
|
List-II |
| A. |
Haber process |
I. |
Fe catalyst |
| B. |
Wacker oxidation |
II. |
\(PdCl_2\) |
| C. |
Wilkinson catalyst |
III. |
\([PPh_3)_3 RhCl]\) |
| D. |
Ziegler catalyst |
IV. |
\(TiCl_4~ \text {with}~ Al(CH_3)_3\) |
Choose the correct answer from the options given below:
1. A-I, B-II, C-III, D-IV
2. A-I, B-IV, C-III, P-II
3. A-I, B-II, C-IV, D-III
4. A-II, B-III, C-I, D-IV
56. Given below are two statements :
| Statement I: |
Like nitrogen that can form ammonia, arsenic can form arsine. |
| Statement II: |
Antimony cannot form antimony pentoxide. |
In light of the above statements, choose the most appropriate answer from the options given below:
| 1. |
Statement I is correct but Statement II is incorrect |
| 2. |
Statement I is incorrect but Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
57. Given below are two statements :
| Statement I: |
Ferromagnetism is considered as an extreme form of paramagnetism. |
| Statement II: |
The number of unpaired electrons in a \(Cr^{2+}\)ion (Z = 24) is the same as that of a \(Nd^{3+}\) ion (Z = 60). |
In the light of the above statements, choose the correct answer from the options given below:
| 1. |
Statement I is True but Statement II is False. |
| 2. |
Statement I is False but Statement II is True. |
| 3. |
Both Statement I and Statement II are True. |
| 4. |
Both Statement I and Statement II are False. |
58. Which one of the following reactions does not give benzene as a product?
59. Match
List-I with
List-II
|
List-I |
|
List-II |
| A. |
\(XeO_3\) |
I. |
\(sp^3d;\) linear |
| B. |
\(XeO_2\) |
II. |
\(sp^3;\) pyramidal |
| C. |
\(XeOF_4\) |
III. |
\(sp^3d^3;\) distorted octahedral |
| D. |
\(XeF_6\) |
IV. |
\(sp^3d^2 ;\) square pyramidal |
Choose the correct answer from the options given below:
| 1. |
A-IV, B-II, C-III, D-I |
2. |
A-IV, B-II, C-I, D-III |
| 3. |
A-II, B-I, C-IV, D-III |
4. |
A-II, B-I, C-III, D-IV |
60. How many products (including stereoisomers) are expected from monochlorination of the following compound?

| 1. |
\(5 \) |
2. |
\(6 \) |
| 3. |
\(2 \) |
4. |
\(3\) |
61. Which of the following statements are true?
| A. |
Unlike Ga that has a very high melting point, Cs has a very low melting point. |
| B. |
On Pauling scale, the electronegativity values of N and Cl are not the same. |
| C. |
\(Ar, K^+, Cl^-, Ca^{2+}\) and \(S^{2-}\) are all isoelectronic species. |
| D. |
The correct order of the first ionization enthalpies of Na, Mg, Al, and Si is Si > Al > Mg > Na |
| E. |
The atomic radius of Cs is greater than that of Li and Rb. |
Choose the correct answer from the options given below:
| 1. |
C and D only |
| 2. |
A, C and E only |
| 3. |
A, B and E only |
| 4. |
C and E only |
62. The standard heat of formation, in kcal/mol of
\(Ba^{2+}\) is :
[Given : standard heat of formation of
\(SO^{2-}_4\) ion (aq)
\(= -216~ \text{kcal/mol}\), standard heat of crystallisation of
\(BaSO_4 (s)= -4.5~\text{ kcal/mol,}\) standard heat of formation of
\(BaSO_4 (s)= -349 ~\text{kcal/mol]}\)
| 1. |
\(+133.0 \) |
2. |
\(+220.5\) |
| 3. |
\(-128.5 \) |
4. |
\(-133.0\) |
63. Match
List-I with
List-II :
|
List-I
(Example) |
|
List-II
(Type of solution) |
| A. |
Humidity |
I. |
Solid in solid |
| B. |
Alloys |
II. |
Liquid in gas |
| C. |
Amalgams |
III. |
Solid in gas |
| D. |
Smoke |
IV. |
Liquid in solid |
Choose the correct answer from the options given below:
1. A-III, B-I, C-IV, D-II
2. A-III, B-II, C-I, D-IV
3. A-II, B-IV, C-I, D-III
4. A-II, B-I, C-IV, D-III
64. \(C(s) + 2 H_2(g) \rightarrow CH_4(g); \Delta H = -74.8~kJ mol^{-1}\)
Which of the following diagrams gives an accurate representation of the above reaction?
[R→reactants; P→products]
65. If a Sugar ' X ' is found in honey, is a keto sugar, exists in \(\alpha\) and \(\beta\) - anomeric forms and is laevorotatory, then find out 'X':
1. Maltose
2. Sucrose
3. D-Glucose
4. D-Fructose
66. The total number of possible isomers (both structural as well as stereoisomers)
of cyclic ethers of molecular formula \(C_4H_8O \) is :
1. 10
2. 11
3. 6
4. 8
67. For the reaction
\(A(g) \rightleftharpoons 2B(g),\) the backward reaction rate constant is higher than the forward reaction rate constant by a factor of
\(2500\) at
\(1000~K \).
\(K_p\) for the reaction at
\(1000~ K\) is:
[ Given:
\({R= 0.0831~ L ~\text{atm mol}^{-1} }K^{-1}]\)
| 1. |
\(0.033 \) |
2. |
\(0.021 \) |
| 3. |
\(83.1 \) |
4. |
\(2 .077 \times 10^5\) |
68. The ratio of the wavelengths of the light absorbed by a Hydrogen atom when it undergoes
\(n=2 \rightarrow n=3\) and
\(n=4 \rightarrow n=6\)
transitions, respectively, is
| 1. |
\(\dfrac{1}{9}\) |
2. |
\(\dfrac{1}{4}\) |
| 3. |
\(\dfrac{1}{36}\) |
4. |
\(\dfrac{1}{16}\) |
69. If the molar conductivity
\(\left(\Lambda_{\mathrm{m}}\right)\) of a
\(0.050 ~\text{mol}~ \text{L}^{-1}\) solution of a monobasic weak acid is
\(90 ~\text{S} ~\text{cm}^2 ~\text{mol}^{-1}\), then its degree of dissociation will be: [Assume
\(\Lambda_{+}^0=349.6~ \mathrm{S ~cm}^2 \mathrm{~mol}^{-1}\) and
\(\mathrm{\Lambda}_{-}^{\circ}=50.4 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1} \)]
| 1. |
\( 0.225 \) |
2. |
\( 0.215 \) |
| 3. |
\(0.115 \) |
4. |
\(0.125\) |
70. Which of the following is true, when
\(5\) moles of liquid
\(\text X\) and
\(10\) moles of liquid
\(\text Y\) make a solution having a vapour pressure of
\(70\) torr?
[Given: The vapour pressures of pure
\(\text X\) and
\(\text Y\) are
\(63\) torr and
\(78\) torr respectively]
| 1. |
The solution is ideal. |
| 2. |
The solution has a volume that is greater than the sum of individual volumes. |
| 3. |
The solution shows positive deviation. |
| 4. |
The solution shows negative deviation. |
71. Consider the following data of compounds:
| A. |
\(212 ~g ~\text{of}~Na_2CO_3 (s) \text{[molar mass}=106 ~g]\) |
| B. |
\(~248 g ~\text{of}~ {Na}_2 \mathrm{O}({s}) [\text{molar mass} =62 \mathrm{~g} ]\) |
| C. |
\(240 g ~\text{of} ~NaOH (s)~ [\text{molar mass} =40 \mathrm{~g} \) |
| D. |
\( 12 g~ \text{of}~ \mathrm{H}_2(\mathrm{~g})[ \text{molar mass} =2 \mathrm{~g}].\) |
| E. |
\( 220 g~ \text{of} ~\mathrm{CO}_2(\mathrm{~g})[\text{ molar mass }=44 \mathrm{~g}]\) |
Which of the following represents a correct set of compounds having an equal number of atoms?
1.
B,
C, and
D only
2.
B,
D, and
E only
3.
A,
B, and
C only
4.
A,
B, and
D only
72. Which of the following are paramagnetic?
| A. |
\(\left[{NiCl}_4\right]^{2-}\) |
B. |
\(Ni(CO)_4\) |
| C. |
\(\left[{Ni}({CN})_4\right]^{2-}\) |
D. |
\(\left[{Ni}\left({H}_2 {O}\right)_6\right]^{2+}\) |
| E. |
\({Ni}\left({PPh}_3\right)_4\) |
Choose the correct answer from the options given below:
| 1. |
A and D only |
2. |
A, D and E only |
| 3. |
A and C only |
4. |
B and E only |
73. If the half-life \((t_{1/2})\) for a first order reaction is \(1~\text{minute}\), then the time required for \(99.9 \%\) completion of the reaction is closest to :
1. \(5~\text{minutes}\)
2. \(10~\text{minutes}\)
3. \(2~\text{minutes}\)
4. \(4~\text{minutes}\)
74. Energy and radius of the first Bohr orbit of
\(He^+\) and
\(Li^{2+}\) are :
[Given
\(\left.\mathrm{R}_{\mathrm{H}}=2.18 \times 10^{-18} \mathrm{~J}, \mathrm{a}_0=52.9 \mathrm{pm}\right]\)
| 1. |
\(\begin{aligned} & \mathrm{E}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=-19.62 \times 10^{-16} \mathrm{~J} ; \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=17.6\mathrm{pm} \\ & \mathrm{E}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=8.72 \times 10^{-16} \mathrm{~J} ; \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=26.4 \mathrm{pm} \end{aligned}\) |
| 2. |
\(\begin{aligned} & \mathrm{E}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=-8.72 \times 10^{-16} \mathrm{~J} ; \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=17.6 \mathrm{pm} \\ & \mathrm{E}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=-19.62 \times 10^{-16} \mathrm{~J} ; \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=17.6 \mathrm{pm} \end{aligned}\) |
| 3. |
\(\begin{aligned} & \mathrm{E}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=- 19.62 \times 10^{-18} \mathrm{~J} \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=17.6 \mathrm{pm} \\ & \mathrm{E}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=-8.72 \times 10^{-18} \mathrm{~J} \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=26.4 \mathrm{pm} \end{aligned}\) |
| 4. |
\(\begin{aligned} & \mathrm{E}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=-8.72 \times 10^{-18} \mathrm{~J} \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{Li}^{2+}\right)=26.4 \mathrm{pm} \\ & \mathrm{E}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=-19.62 \times 10^{-18} \mathrm{~J} ; \\ & \mathrm{r}_{\mathrm{n}}\left(\mathrm{He}^{+}\right)=17.6 \mathrm{pm} \end{aligned} \) |
75. Among the given compounds I-III, the correct order of bond dissociation energy of
\(C-H\) bond marked with * is:
1. III > II > I
2. II > III > I
3. II > I > III
4. I > II > III
76. Dalton's Atomic theory could not explain which of the following?
1. Law of multiple proportion
2. Law of gaseous volume
3. Law of conservation of mass
4. Law of constant proportion
77. Identify the correct orders against the property mentioned:
| A. |
\(\mathrm{H}_2 \mathrm{O}>\mathrm{NH}_3>\mathrm{CHCl}_3 -\text { }\)dipole moment |
| B. |
\(\mathrm{XeF}_4>\mathrm{XeO}_3>\mathrm{XeF}_2-\text { }\)number of lone pairs on central atom |
| C. |
\(\mathrm{O}-\mathrm{H}>\mathrm{C}-\mathrm{H}>\mathrm{N}-\mathrm{O}-\text { }\)bond length |
| D. |
\(\mathrm{N}_2>\mathrm{O}_2>\mathrm{H}_2 \text { } -\) bond enthalpy |
Choose the correct answer from the options given below:
1.
A and
C only
2.
B and
C only
3.
A and
D only
4.
B and
D only
78. Match
List-I with
List-II.
|
List-I
(Name of Vitamin) |
|
List-II
(Deficiency disease) |
| A. |
Vitamin \(B_{12}\) |
I. |
Cheilosis |
| B. |
Vitamin \(D\) |
II. |
Convulsions |
| C. |
Vitamin \(B_{2}\) |
III. |
Rickets |
| D. |
Vitamin \(B_{6}\) |
IV. |
Pernicious anaemia |
Choose the correct answer from the options given below:
1. A-II, B-III, C-I, D-IV
2. A-IV, B-III, C-II, D-I
3. A-I, B-III, C-II, D-IV
4. A-IV, B-III, C-I, D-II
79. The correct order of decreasing basic strength of the given amines is:
| 1. |
N-ethylethanamine > ethanamine > N -methylaniline > benzenamine |
| 2. |
benzenamine > ethanamine > N -methylaniline >N-ethylethanamine |
| 3. |
N -methylaniline > benzenamine > ethanamine >N-ethylethanamine |
| 4. |
N-ethylethanamine > ethanamine > benzenamine >N-methylaniline |
80. The correct order of the wavelength of light absorbed by the following complexes is.
| A. |
\(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+} \) |
B. |
\(\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-} \) |
| C. |
\(\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{2+} \) |
D. |
\(\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\) |
Choose the correct answer from the options given below:
| 1. |
\(C<D<A<B\) |
2. |
\(C<A<D<B\) |
| 3. |
\(B<D<A<C\) |
4. |
\(B<A<D<C\) |
81. Which one of the following compounds does not decolourize bromine water?
82. Predict the major product
\('P'\) in the following sequence of reactions:
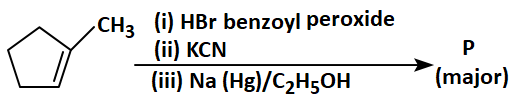
83. Match
List-I with
List-II:
|
List-I
(Mixture) |
|
List-II
(Method of Separation) |
| A. |
\( \mathrm{CHCl}_3+ \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2 \) |
I. |
Distillation under reduced pressure |
| B. |
Crude oil in petroleum industry |
II. |
Steam distillation |
| C. |
Glycerol from spent-lye |
III. |
Fractional distillation |
| D. |
Aniline-water |
Iv. |
Simple distillation |
Choose the correct answer from the option given below:
1. A-III, B-IV, C-I, D-II
2. A-III, B-IV, C-II, D-I
3. A-IV, B-III, C-I, D-II
4. A-IV, B-III, C-II, D-I
84. Which of the following electronic configurations belong to the main group elements?
| A |
\([\mathrm{Ne}] 3 \mathrm{~s}^1\) |
B |
\([\mathrm{Ar}] 3d^3 \mathrm{~4s}^2\) |
| C |
\([\mathrm{Kr}] 4 \mathrm{~d}^{10} 5 \mathrm{~s}^2 5 \mathrm{p}^5\) |
D |
\([\mathrm{Ar}] 3 \mathrm{~d}^{10} 4 \mathrm{~s}^1\) |
| E |
\(\mathrm{[R n] }5 f^0 6 d^2 7 s^2\) |
Choose the correct answer from the option given below:
| 1. |
D and E only |
2. |
A, C and D only |
| 3. |
B and E only |
4. |
A and C only |
85. Which one of the following compounds can exist as cis-trans isomers?
1. 1, 1-Dimethycyclopropane
2. 1, 2-Dimethylcyclohexane
3. Pent-1-ene
4. 2-Methylhex-2-ene
86. Phosphoric acid ionizes in three steps, with their ionization constant values
\(\mathrm{K}_{\mathrm{a}_1}, \mathrm{~K}_{\mathrm{a}_2} \text { and } \mathrm{K}_{\mathrm{a}_3} \text {, }\)respectively, while
\(K\) is the overall ionization constant. Which of the following statements are true for the ionization of phosphoric acid?
| A. |
\(\log \mathrm{K}=\log \mathrm{K}_{\mathrm{a}_1}+\log \mathrm{K}_{\mathrm{a}_2}+\log \mathrm{K}_{\mathrm{a}_3}\) |
| B. |
\(\mathrm{H}_3 \mathrm{PO}_4\) is a stronger acid than \(\mathrm{H}_2 \mathrm{PO}_4^{-}\) and \(\mathrm{HPO}_4^{2-} .\) |
| C. |
\(\mathrm{K}_{a_1} >\mathrm{~K}_{\mathrm{a}_2}>\mathrm{K}_{\mathrm{a}_3} \) |
| D. |
\(\mathrm{K}_{\mathrm{a}_1}=\frac{\mathrm{K}_{\mathrm{a}_3}+\mathrm{K}_{\mathrm{a}_2}}{2}\) |
Choose the correct answer from the options given below :
1.
B,
C and
D only
2.
A,
B and
C only
3.
A and
B only
4.
A and
C only
87. Match List-I with List-II:
List-I
(Ion) |
List-II
(Group Number in Cation Analysis) |
| A. |
\(Co^{2+}\) |
I. |
Group-I |
| B. |
\(Mg^{2+}\) |
II. |
Group-III |
| C. |
\(Pb^{2+}\) |
III. |
Group-IV |
| D. |
\(Al^{3+}\) |
IV. |
Group-VI |
Choose the
correct answer from the options given below:
1. A-III, B-II, C-IV, D-I
2. A-III, B-II, C-I, D-IV
3. A-III, B-IV, C-II, D-I
4. A-III, B-IV, C-I, D-II
88. Which of the following parameters can determine the higher yield of \(\mathrm{NO}\) in the reaction, \(\mathrm{N}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NO}(\mathrm{g})\) ?
\([ Given: \Delta \mathrm{H}\) of the reaction \(=+180.7 \mathrm{~kJ} \mathrm{~mol}^{-1} ]\)
A. higher temperature
B. lower temperature
C. higher concentration of \(\mathrm{N}_2\)
D. higher concentration of \(\mathrm{O}_2\)
Choose the correct answer from the options given below:
1. B, C, D only
2. A, C, D only
3. A, D only
4. B, C only
89. Given below are two statements:
| Statement I: |
Benzenediazonium salt is prepared by the reaction of aniline with nitrous acid at \(273-278~ \text{K}.\) It decomposes easily in the dry state. |
| Statement II: |
Insertion of iodine into the benzene ring is difficult, and hence iodobenzene is prepared through the reaction of benzenediazonium salt with \(KI.\) |
| 1. |
Statement I is correct; Statement II is incorrect |
| 2. |
Statement I is incorrect; Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
90. The major product of the following reaction is:

BIOLOGY
91. 
In the above-represented plasmid, an alien piece of DNA is inserted at EcoRI site. Which of the following strategies will be chosen to select the recombinant colonies?
| 1. |
White color colonies will be selected. |
| 2. |
Blue color colonies grown on ampicillin plates can be selected. |
| 3. |
Using an ampicillin- & tetracycline-containing medium plate. |
| 4. |
Blue color colonies will be selected. |
92. The protein portion of an enzyme is called:
| 1. |
Apoenzyme |
2. |
Prosthetic group |
| 3. |
Cofactor |
4. |
Coenzyme |
93. Given below are two statements:
| Statement I: |
The primary source of energy in an ecosystem is solar energy. |
| Statement II: |
The rate of production of organic matter photosynthesis in an ecosystem is called net primary productivity (NPP).
|
In the light of the above statements, choose the most appropriate answer from this options given below.
| 1. |
Statement I is correct but statement II is incorrect |
| 2. |
Statement I is incorrect but statement II is correct |
| 3. |
Both statement I and statement II are correct |
| 4. |
Both statement I and statement II are incorrect |
94. Given below are two statements: One is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
A typical unfertilised, angiosperm embryo sac at maturity is 8-nucleate and 7-celled. |
| Reason (R): |
The egg apparatus has 2 polar nuclei. |
In the light of the above statements, choose the correct from the options given below:
| 1. |
(A) is True but (R) is False. |
| 2. |
(A) is False but (R) is True. |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A) |
| 4. |
Both (A) and (R) are true but (R) is not the correct explanation of (A) |
95. Neoplastic characteristics of cells refer to:
| A. |
A mass of proliferating cell |
| B. |
Rapid growth of cells |
| C. |
Invasion and damage to the surrounding tissue |
| D. |
Those confined to original location |
Choose the correct answer from the options given below.
| 1. |
A, B, D only |
2. |
B, C, D only |
| 3. |
A, B only |
4. |
A, B, C only |
96. Which one of the following is the characteristic feature of gymnosperms?
| 1. |
Seeds are absent. |
| 2. |
Gymnosperms have flowers for reproduction. |
| 3. |
Seeds are enclosed in fruits. |
| 4. |
Seeds are naked. |
97. Match
List- I with
List-II.
|
List-I |
|
List-II |
| A. |
Progesterone |
I. |
Pars intermedia |
| B. |
Relaxin |
II. |
Ovary |
| C. |
Melanocyte stimulating hormone |
III. |
Adrenal Medulla |
| D. |
Catecholamines |
IV. |
Corpus luteum |
Choose the correct answer from the options given below.
1. A-II, B-IV, C-I, D-III
2. A-III, B-II, C-IV, D-I
3. A-IV, B-II, C-I, D-III
4. A-IV, B-II, C-III, D-I
98. Which chromosome in the human genome has the highest number of genes?
| 1. |
Chromosome 1 |
2. |
Chromosome 10 |
| 3. |
Chromosome X |
4. |
Chromosome Y |
99. Which of the following statements about RuBisCO is true?
| 1. |
It is an enzyme involved in the photolysis of water. |
| 2. |
It catalyzes the carboxylation of RuBP, |
| 3. |
It is active only in the dark. |
| 4. |
It has higher affinity for oxygen than carbon dioxide. |
100. The first menstruation is called:
| 1. |
Diapause |
2. |
Ovulation |
| 3. |
Menopause |
4. |
Menarche |
101. Which of the following genetically engineered organisms was used by Eli Lilly to prepare human insulin?
| 1. |
Virus |
2. |
Phage |
| 3. |
Bacterium |
4. |
Yeast |
102. Given below are two statements: one is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
All vertebrates are chordates but all chordates are not vertebrate. |
| Reason (R): |
The members of subphylum vertebrata possess notochord during the embryonic period, the notochord is replaced by a cartilaginous or bony vertebral column in adults. |
In the light of the above statements, choose the correct answer from the options given below :
| 1. |
(A) is True but (R) is False. |
| 2. |
(A) is False but (R) is True. |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 4. |
Both (A) and (R) are True but (R) is not the correct explanation of (A) |
103. What is the main function of the spindle fibers during mitosis?
| 1. |
To repair damaged DNA |
| 2. |
To regulate cell growth |
| 3. |
To separate the chromosomes |
| 4. |
To synthesize new DNA |
104. Match
List-I with
List-II:
|
List-I |
|
List-II |
| A. |
Alfred Hershey and Martha Chase |
I. |
Streptococcus pneumoniae |
| B. |
Euchromatin |
II. |
Densely packed and dark- stained |
| C. |
Frederick Griffith |
III. |
Loosely packed and light-stained |
| D. |
Heterochromatin |
IV. |
DNA as genetic material confirmation |
Choose the correct answer from the options given below:
| 1. |
A-IV, B-III, C-I, D-II |
2. |
A-III, B-II, C-IV, D-I |
| 3. |
A-II, B-IV, C-I, D-III |
4. |
A-IV, B-II, C-I, D-III |
105. Match
List-I with
List-II:
|
List-I |
|
List-II |
| A. |
Adenosine |
I. |
Nitrogen base |
| B. |
Adenylic acid |
II. |
Nucleotide |
| C. |
Adenine |
III. |
Nucleoside |
| D. |
Alanine |
IV. |
Amino acid |
Choose the option with all correct matches.
| 1. |
A-III, B-II, C-I, D-IV |
2. |
A-II, B-III, C-I, D-IV |
| 3. |
A-III, B-IV, C-II, D-I |
4. |
A-III, B-II, C-IV, D-I |
106. In frog, the Renal portal system is a special venous connection that acts to link:
1. Kidney and intestine
2. Kidney and lower part of the body
3. Liver and intestine
4. Liver and kidney
107. Which of the following are the post-transcriptional events in an eukaryotic cell?
| A. |
Transport of pre-mRNA to the cytoplasm prior to splicing |
| B. |
Removal of introns and joining of exons. |
| C. |
Addition of methyl group at 5' end of hnRNA. |
| D. |
Addition of adenine residues at 3' end of hnRNA. |
| E. |
Base pairing of two complementary RNAs. |
Choose the correct answer from the options given below:
| 1. |
B, C, E only |
2. |
C, D, E only |
| 3. |
A, B, C only |
4. |
B, C, D only |
108. Polymerase chain reaction (PCR) amplifies DNA following the equation.
| 1. |
\(2n+1\) |
2. |
\(2N^2\) |
| 3. |
\(N^2\) |
4. |
\(2^n\) |
109. Given below are two statements: One is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
Both wind and water pollinated flowers are not very colourful and do not produce nectar. |
| Reason (R): |
The flowers produce enormous amount of pollen grains in wind and water pollinated flowers. |
In the light of the above statements, choose the correct answer from the options given below.
| 1. |
(A) is True but (R) is False. |
| 2. |
(A) is False but (R) is True. |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 4. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
110. Epiphytes, that are growing on the mango branch, is an explanation of which of the following?
| 1. |
Predation |
2. |
Amensalism |
| 3. |
Commensalism |
4. |
Mutualism |
111. Find the correct statements:
| A. |
In human pregnancy, the major organ systems are formed at the end of 12 weeks. |
| B. |
In human pregnancy, the major organ systems are formed at the end of 8 weeks. |
| C. |
In human pregnancy, the heart is formed after one month of gestation. |
| D. |
In human pregnancy, limbs and digits develop by the end of the second month. |
| E. |
In human pregnancy, the appearance of hair is usually observed in the fifth month. |
Choose the correct answer from the options given below:
| 1. |
B, C, D and E only |
| 2. |
A, C, D and E Only |
| 3. |
A and E Only |
| 4. |
B and C Only |
112. Identify the part of a bio-reactor which is used as a foam braker from the given figure.

113. Frogs respire in water by skin and buccal cavity and on land by skin, buccal cavity and lungs.
Choose the correct answer from the following:
| 1. |
The statement is false for water but true for land |
| 2. |
The statement is false for both the environments |
| 3. |
The statement is true for water but false for land |
| 4. |
The statement is true for both the environments |
114. Consider the following statements regarding functions of adrenal medullary hormones:
| A. |
It causes pupillary constriction. |
| B. |
It is a hyperglycemic hormone. |
| C. |
It causes piloerection. |
| D. |
It increases strength of heart contraction. |
Choose the correct answer from the options given below.
| 1. |
A, C and D only |
2. |
D only |
| 3. |
C and D only |
4. |
B, C and D only |
115. Read the following statements on plant growth and development.
| A. |
Parthenocarpy can be induced by auxins. |
| B. |
Plant growth regulators can be involved in promotion as well as inhibition of growth |
| C. |
Dedifferentiation is a pre-requisite for re-differentiation. |
| D. |
Abscisic acid is a plant growth promoter. |
| E. |
Apical dominance promotes the growth of lateral buds. |
Choose the option with all correct statements:
| 1. |
A, D, E only |
| 2. |
B, D, E only |
| 3. |
A, B, C only |
| 4. |
A, C, E only |
116. Which of the following hormones released from the pituitary is actually synthesized in the hypothalamus?
| 1. |
Follicle-stimulating hormone (FSH) |
| 2. |
Adrenocorticotrophic hormone (ACTH) |
| 3. |
Luteinizing hormone (LH) |
| 4. |
Anti-diuretic hormone (ADH) |
117. Which of the following is an example of a non-distilled alcoholic beverage produced by yeast?
| 1. |
Beer |
2. |
Rum |
| 3. |
Whisky |
4. |
Brandy |
118.
What is the pattern of inheritance for polygenic traits?
| 1. |
Autosomal dominant pattern |
| 2. |
X-linked recessive inheritance pattern |
| 3. |
Mendelian inheritance pattern |
| 4. |
Non-Mendelian inheritance pattern |
119. Match
List-I with
List-II
|
List-I |
|
List-II |
| A. |
Head |
I. |
Enzymes |
| B. |
Middle piece |
II. |
Sperm motility |
| C. |
Acrosome |
III. |
Energy |
| D. |
Tail |
IV. |
Genetic materials |
Choose the correct answer from the options given below :
1. A-III, B-IV, C-II, D-I
2. A-III, B-II, C-I, D-IV
3. A-IV, B-III, C-I, D-II
4. A-IV, B-III, C-II, D-I
120. Which of the following is an example of a zygomorphic flower ?
1. Pea
2. Chilli
3. Petunia
4. Datura
121. Which of the following organisms
cannot fix nitrogen?
| A. |
Azotobacter |
B. |
Oscillatoria |
| C. |
Anabaena |
D. |
Volvox |
| E. |
Nostoc |
Choose the correct answer from the options given below:
| 1. |
B only |
2. |
E only |
| 3. |
A only |
4. |
D only |
122. Which one of the following is an example of ex-situ conservation?
1. Zoos and botanical gardens
2. Protected areas
3. National Park
4. Wildlife Sanctuary
123. Who is known as the father of Ecology in India?
1. Ram Udar
2. Birbal Sahni
3. S. R. Kashyap
4. Ramdeo Misra
124. Given below are two statements :
| Statement I : |
In the RNA world, RNA is considered the first genetic material evolved to carry out essential life processes. RNA acts as a genetic material and also as a catalyst for some important biochemical reactions in living systems. Being reactive, RNA is unstable. |
| Statement II : |
DNA evolved from RNA and is a more stable genetic material. Its double helical strands being complementary, resist changes by evolving repairing mechanism. |
| 1. |
Statement I is correct but Statement II is incorrect |
| 2. |
Statement I is incorrect but Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
125. Given below are two statements :
| Statement I: |
Transfer RNAs and ribosomal RNA do not interact with mRNA. |
| Statement II: |
RNA interference (RNAi) takes place in all eukaryotic organisms as a method of cellular defence. |
In the light of the above statements, choose the most appropriate answer from the options given below:
| 1. |
Statement I is correct but Statement II is incorrect |
| 2. |
Statement I is incorrect but Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
126. Match the
List-I with
List-II
|
List-I |
|
List-II |
| A. |
Heart |
I. |
Erythropoietin |
| B. |
Kidney |
II. |
Aldosterone |
| C. |
Gastro - intestinal tract |
III. |
Atrial natriuretic factor |
| D. |
Adrenal Cortex |
IV. |
Secretin |
Choose the correct answer from the options given below:
1. A-I, B-III, C-IV, D-II
2. A-III, B-I, C-IV, D-II
3. A-II, B-I, C-III, D-IV
4. A-IV, B-III, C-II, D-I
127. All living members of the class Cyclostomata are:
1. Symbiotic
2. Ectoparasite
3. Free living
4. Endoparasite
128. Streptokinase produced by bacterium
Streptococcus is used for
| 1. |
Liver disease treatment |
| 2. |
Removing clots from blood vessels |
| 3. |
Curd production |
| 4. |
Ethanol production |
129. Role of the water vascular system in Echinoderms is:
| A. |
Respiration and locomotion |
| B. |
Excretion and locomotion |
| C. |
Capture and transport of food |
| D. |
Digestion and respiration |
| E. |
Digestion and excretion |
Choose the correct answer from the options given below:
1.
B and
C only
2.
B, D and
E only
3.
A and
B only
4.
A and
C only
130. Match
List-I with
List-II
|
List-I |
|
List-II |
| A. |
Pteridophyte |
I. |
Salvia |
| B. |
Bryophyte |
II. |
Ginkgo |
| C. |
Angiosperm |
III. |
Polystrichum |
| D. |
Gymnosperm |
IV. |
Salvinia |
Choose the option with all correct matches.
| 1. |
A-III, B-IV, C-I, D-II |
2. |
A-IV, B-III, C-II, D-I |
| 3. |
A-III, B-IV, C-II, D-I |
4. |
A-IV, B-III, C-I, D-II |
131. Which are correct:
| A. |
Computed tomography and magnetic resonance imaging detect cancers of internal organs. |
| B. |
Chemotherapeutic drugs are used to kill non-cancerous cells. |
| C. |
\(\alpha\) Interferon activates the cancer patients' immune system and helps in destroying the tumor. |
| D. |
Chemotherapeutic drugs are biological response modifiers. |
| E. |
In the case of leukemia, blood cell counts decrease. |
Choose the correct answer from the options given below:
| 1. |
C and D only |
2. |
A and C only |
| 3. |
B and D only |
4. |
D and E only |
132. What are the potential drawbacks in adoption of the IVF method?
| A. |
High fatality risk to mother |
| B. |
Expensive instruments and reagents |
| C. |
Husband/wife necessary for being donors |
| D. |
Less adoption of orphans |
| E. |
Not available in India |
| F. |
Possibility that the early embryo does not survive |
Choose the correct answer from the options given below:
| 1. |
A, B, C, D only |
2. |
A, B, C, E, F only |
| 3. |
B, D, F only |
4. |
A, C, D, F only |
133. Consider the following:
| A. |
The reductive division for the human female gametogenesis starts earlier than that of the male gametogenesis. |
| B. |
The gap between the first meiotic division and the second meiotic division is much shorter for males compared to females. |
| C. |
The first polar body is associated with the formation of the primary oocyte. |
| D. |
Luteinizing Hormone (LH) surge leads to disintegration of the endometrium and onset of menstrual bleeding. |
Choose the correct answer from the options given below:
1.
B and
D are true
2.
B and
C are true
3.
A and
B are true
4.
A and
C are true
134. In bryophytes, the gemmae help in which one of the following?
| 1. |
Nutrient absorption |
2. |
Gaseous exchange |
| 3. |
Sexual reproduction |
4. |
Asexual reproduction |
135. Given below are two statements : one is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
The primary function of the Golgi apparatus is to package the materials made by the endoplasmic reticulum and deliver it to intracellular targets and outside the cell. |
| Reason (R): |
Vesicles containing materials made by the endoplasmic reticulum fuse with the cis face of the Golgi apparatus, and they are modified and released from the trans face of the Golgi apparatus. |
In the light of the above statements, choose the correct answer from the options given below :
| 1. |
(A) is True but (R) is False. |
| 2. |
(A) is False but (R) is True. |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 4. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
136. Which one of the following statements refers to Reductionist Biology?
| 1. |
Chemical approach to study and understand living organisms |
| 2. |
Behavioural approach to study and understand living organisms |
| 3. |
Physico-chemical approach to study and understand living organisms |
| 4. |
Physiological approach to study and understand living organisms |
137. After maturation, in primary lymphoid organs, the lymphocytes migrate for interaction with antigens to secondary lymphoid organ(s) / tissue(s) like:
| A. |
thymus |
| B. |
bone marrow |
| C. |
spleen |
| D. |
lymph nodes |
| E. |
Peyer's patches |
Choose the correct answer from the options given below:
| 1. |
E, A, B only |
2. |
C, D, E only |
| 3. |
B, C, D only |
4. |
A, B, C only |
138. Match
List-I with
List-II:
|
List-I |
|
List-II |
| A. |
The Evil Quartet |
I. |
Cryopreservation |
| B. |
Ex-situ |
II. |
Alien species invasion |
| C. |
Lantana camara |
III. |
Causes of biodiversity losses |
| D. |
Dodo |
IV. |
Extinction |
Choose the options with all correct matches.
1. A-III, B-IV, C-II, D-I
2. A-III, B-II, C-IV, D-I
3. A-III, B-II, C-I, D-IV
4. A-III, B-I, C-II, D-IV
139. How many meiotic and mitotic divisions need to occur for the development of a mature female gametophyte from the megaspore mother cell in an angiosperm plant?
1. 1 Meiosis and 3 Mitosis
2. No Meiosis and 2 Mitosis
3. 2 Meiosis and 3 Mitosis
4. 1 Meiosis and 2 Mitosis
140. Which of the following type of immunity is present at the time of birth and is a non-specific type of defence in the human body?
1. Cell-mediated Immunity
2. Humoral Immunity
3. Acquired Immunity
4. Innate Immunity
141. Given below are two statements :
| Statement I: |
Fig fruit is a non-vegetarian fruit as it has enclosed fig wasps in it. |
| Statement II: |
Fig wasp and fig tree exhibit mutual relationship as fig wasp completes its life cycle in fig fruit and fig fruit gets pollinated by fig wasp. |
In the light of the above statements, choose the most appropriate answer from the options given below:
| 1. |
Statement I is correct but Statement II is incorrect |
| 2. |
Statement I is incorrect but Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
142. Given below are two statements: one is labelled as
Assertion (A) and the other is labelled as
Reason (R).
| Assertion (A): |
Cells of the tapetum possess dense cytoplasm and generally have more than one nucleus. |
| Reason (R): |
The presence of more than one nucleus in the tapetum increases the efficiency of nourishing the developing microspore mother cells. |
In light of the above statement, choose the most appropriate answer from the options given below:
| 1. |
(A) is True but (R) is False. |
| 2. |
(A) is False but (R) is True. |
| 3. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 4. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
143. From the statements given below, choose the correct option:
| A. |
The eukaryotic ribosomes are 80S and prokaryotic ribosomes are 70S. |
| B. |
Each ribosome has two subunits. |
| C. |
The two sub-units of 80S ribosomes are 60S and 40S, while those of 70S are 50S and 30S |
| D. |
The two subunits of 80S ribosomes are 60S and 20S, and those of 70S are 50S and 20S |
| E. |
The two subunits of 80S are 60S and 30S, and those of 70S are 50S and 30S. |
| 1. |
A, B and E are true |
2. |
B, D and E are true |
| 3. |
A, B and C are true |
4. |
A, B and D are true |
144. Which one of the following enzymes contains 'Haem' as the prosthetic group?
1. Succinate dehydrogenase
2. Catalase
3. RuBisCo
4. Carbonic anhydrase
145. What is the name of the blood vessel that carries deoxygenated blood from the body to the heart in a frog?
1. Pulmonary vein
2. Vena cava
3. Aorta
4. Pulmonary artery
146. Given below are the stages in the life cycle of pteridophytes. Arrange the following stages in the correct sequence.
| A. |
Prothallus stage |
| B. |
Meiosis in spore mother cells |
| C. |
Fertilisation |
| D. |
Formation of archegonia and antheridia in gametophyte. |
| E. |
Transfer of antherozoids to the archegonia in presence of water. |
Choose the correct answer from the options given below.
| 1. |
D, E, C, A and B |
2. |
E, D, C, B and A |
| 3. |
B, A, D, E and C |
4. |
B, A, E, C and D |
147. The blue and white selectable markers have been developed which differentiate recombinant colonies from non-recombinant colonies on the basis of their ability to produce colour in the presence of a chromogenic substrate.
Given below are two statements about this method:
| Statement I: |
The blue coloured colonies have DNA insert in the plasmid and they are identified as recombinant colonies. |
| Statement II: |
The colonies without blue colour have DNA insert in the plasmid and are identified as recombinant colonies. |
| 1. |
Statement I is correct; Statement II is incorrect |
| 2. |
Statement I is incorrect; Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
148. Which of the following microbes is NOT involved in the preparation of household products?
| A. |
Aspergillus niger |
| B. |
Lactobacillus |
| C. |
Trichoderma polysporum |
| D. |
Saccharomyces cerevisiae |
| E. |
Propionibacterium sharmanii |
Choose the correct answer from the options given below:
| 1. |
C and D only |
2. |
C and E only |
| 3. |
A and B only |
4. |
A and C only |
149. Silencing of specific mRNA is possible via RNAi because of -
1. Complementary tRNA
2. Non-complementary ssRNA
3. Complementary dsRNA
4. Inhibitory ssRNA
150. The complex II of mitochondrial electron transport chain is also known as
1. Cytochrome c oxidase
2. NADH dehydrogenase
3. Cytochrome bc
4. Succinate dehydrogenase
151. While trying to find out the characteristic of a newly found animal, a researcher did the histology of adult animal and observed a cavity with presence of mesodermal tissue towards the body wall but no mesodermal tissue was observed towards the alimentary canal. What could be the possible coelome of that animal?
| 1. |
Schizocoelomate |
2. |
Spongocoelomate |
| 3. |
Acoelomate |
4. |
Pseudocoelomate |
152. Given below are two statements :
| Statement I : |
In a floral formula, \(\oplus\) stands for zygomorphic nature of the flower, and G stands for inferior ovary. |
| Statement II: |
In a floral formula, \(\oplus\) stands for actinomorphic nature of the flower and G stands for superior ovary. |
In the light of the above statements, choose the correct answer from the options given below:
| 1. |
Statement I is correct but Statement II is incorrect |
| 2. |
Statement I is incorrect but Statement II is correct |
| 3. |
Both Statement I and Statement II are correct |
| 4. |
Both Statement I and Statement II are incorrect |
153. Given below are two statements:
| Statement I: |
In an ecosystem, there is unidirectional flow of energy of sun from producers to consumers. |
| Statement II: |
Ecosystems are exempted from 2nd law of thermodynamics. |
In the light of the above statements, choose the most appropriate answer from the options given below:
| 1. |
Statement I is correct but statement II is incorrect |
| 2. |
Statement I is incorrect but statement II is correct |
| 3. |
Both statement I and statement II are correct |
| 4. |
Both statement I and statement II are incorrect |
154. Which of the following is the unit of productivity of an ecosystem?
1. \(\mathrm{KCal} \mathrm{~m}^{-3} \)
2. \(\left(\mathrm{KCal} \mathrm{~m}^{-2}\right) \mathrm{yr}^{-1} \)
3. \(\mathrm{gm}^{-2}\)
4. \(\mathrm{KCal} \mathrm{~m}^{-2}\)
155. With the help of the given pedigree, find out the probability for the birth of a child having no disease and being a carrier (has the disease mutation in one allele of the gene) in
\(F_3\) generation.

1. 1/8
2. Zero
3. 1/4
4. 1/2
156. In the seeds of cereals, the outer covering of endosperm separates the embryo by a protein-rich layer called:
| 1. |
Integument |
2. |
Aleurone layer |
| 3. |
Coleoptile |
4. |
Coleorhiza |
157.
Match List-I with List-II:
|
List-I |
|
List-II |
| A. |
Chlorophyll a |
I. |
Yellow-green |
| B. |
Chlorophyll b |
II. |
Yellow |
| C. |
Xanthophylls |
III. |
Blue-green |
| D. |
Carotenoids |
IV. |
Yellow to Yellow-orange |
Choose the option with all correct matches:
1. A-I, B-II, C-IV, D-III
2. A-I, B-IV, C-III, D-II
3. A-III, B-IV, C-II, D-I
4. A-III, B-I, C-II, D-IV
158. Who proposed that the genetic code for amino acids should be made up of three nucleotides?
1. Jacque Monod
2. Franklin Stahl
3. George Gamow
4. Francis Crick
159. Histones are enriched with
1. Phenylalanine & Leucine
2. Phenylalanine & Arginine
3. Lysine & Arginine
4. Leucine & Lysine
160. Which of the following enzyme(s) are NOT essential for gene cloning?
A. Restriction enzymes
B. DNA ligase
C. DNA mutase
D. DNA recombinase
E. DNA polymerase
Choose the correct answer from the options given below:
| 1. |
D and E only |
2. |
B and C only |
| 3. |
C and D only |
4. |
A and B only |
161. A specialised membranous structure in a prokaryotic cell which helps in cell wall formation, DNA replication is :
1. Cristae
2. Endoplasmic Reticulum
3. Mesosome
4. Chromatophores
162. Which factor is important for termination of transcription?
| 1. |
\(\rho\) (rho) |
2. |
\(\gamma\) (gamma) |
| 3. |
\(\alpha\) (alpha) |
4. |
\(\sigma\) (sigma) |
163. Which of the following statement is correct about location of the male from copulatory pad?
1. Second digit of fore limb
2. First digit of the fore limb
3. First and Second digit of fore limb
4. First digit of hind limb
164. Which of the following diagrams is correct with regard to the proximal (P) and distal (D) tubule of the Nephron.
165. Identify the statement that is NOT correct.
| 1. |
Antigen binding site is located at C-terminal region of antibody molecules. |
| 2. |
Constant region of heavy and light chains are located at C-terminus of antibody molecules. |
| 3. |
Each antibody has two light and two heavy chain. |
| 4. |
The heavy and light chains are held together by disulfide bonds. |
166. Match
List-I with
List-II:
|
List-I |
|
List-II |
| A. |
Scutellum |
I. |
Persistent nucellus |
| B. |
Non-albuminous |
II. |
Cotyledon of monocot seed |
| C. |
Epiblast |
III. |
Groundnut |
| D. |
Perisperm |
IV. |
Rudimentary cotyledon |
Choose the option with all correct matches.
1. A-IV, B-III, C-I, D-II
2. A-II, B-IV, C-III, D-I
3. A-II, B-III, C-IV, D-I
4. A-IV, B-III, C-II, D-I
167. Find the statement that is NOT correct with regard to the structure of monocot stem.
| 1. |
Vascular bundles are conjoint and closed. |
| 2. |
Phloem parenchyma is absent. |
| 3. |
Hypodermis is parenchymatous. |
| 4. |
Vascular bundles are scattered. |
168. Twins are born to a family that lives next door to you. The twins are a boy and a girl. Which of the following must be true?
| 1. |
The were conceived through in vitro fertilization. |
| 2. |
They have 75% identical genetic content. |
| 3. |
They are monozygotic twins. |
| 4. |
They are fraternal twins. |
169. Sweet potato and potato represent a certain type of evolution. Select the correct combination of terms to explain the evolution.
| 1. |
Homology, convergent |
2. |
Analogy, divergent |
| 3. |
Analogy, convergent |
4. |
Homology, divergent |
170. Which one of the following phytohormones promotes nutrient mobilization which helps in the delay of leaf senescence in plants?
| 1. |
Gibberellin |
2. |
Cytokinin |
| 3. |
Ethylene |
4. |
Abscisic acid |
171. Why can't insulin be given orally to diabetic patients?
| 1. |
Because of structural variation |
| 2. |
Its bioavailability will be increased |
| 3. |
Human body will elicit strong immune response |
| 4. |
It will be digested in Gastro-Intestinal (GI) tract |
172. Name the class of enzyme that usually catalyze the following reaction :
\(\mathrm{S}-\mathrm{G}+\mathrm{S}^{\#} \rightarrow \mathrm{~S}+\mathrm{S}^{\#}-\mathrm{G}\)
Where, G → a group other than hydrogen
S → a substrate
\(S^\#\) → another substate
1. Transferase
2. Ligase
3. Hydrolase
4. Lyase
173. Given below are two statements :
| Statement I : |
The DNA fragments extracted from gel electrophoresis can be used in construction of recombinant DNA. |
| Statement II : |
Smaller size DNA fragments are observed near anode while larger fragments are found near the wells in an agarose gel. |
In the light of the above statements, choose the most appropriate answer from the options given below :
| 1. |
Statement I is correct but statement II is incorrect |
| 2. |
Statement I is incorrect but statement II is correct |
| 3. |
Both statement I and statement II are correct |
| 4. |
Both statement I and statement II are incorrect |
174. The correct sequence of events in the life cycle of bryophytes is
A. Fusion of antherozoid with egg.
B. Attachment of gametophyte to substratum.
C. Reduction division to produce haploid spores.
D. Formation of sporophyte.
E. Release of antherozoids into water.
Choose the correct answer from the options given below:
| 1. |
B, E, A, D, C |
2. |
D, E, A, B, C |
| 3. |
D, E, A, C, B |
4. |
B, E, A, C, D |
175. Genes R and Y follow independent assortment. If RRYY produce round yellow seed and rryy produce wrinkled green seeds, what will be then phenotypic ratio of the F2 generation?
1. Phenotypic ratio - 9 : 3 : 3 : 1
2. Phenotypic ratio - 9 :7
3. Phenotypic ratio - 1 : 2 : 1
4. Phenotypic ratio - 3 : 1
176. Each of the following characteristics represent a Kingdom proposed by Whittaker. Arrange the following in increasing order of complexity of body organization.
| A. |
Multicellular heterotrophs with cell wall made of chitin. |
| B. |
Heterotrophs with tissue/organ/organ system level of body organization. |
| C. |
Prokaryotes with cell wall made of polysaccharides and amino acids. |
| D. |
Eukaryotic autotrophs with tissue/organ level of body organization. |
| E. |
Eukaryotes with cellular body organization. |
Choose the correct answer from the options given below :
| 1. |
A, C, E, D, B |
2. |
C, E, A, B, D |
| 3. |
A, C, E, B, D |
4. |
C, E, A, D, B |
177. Match
List-I with
List-II.
|
List-I |
|
List-I |
| A. |
Centromere |
I. |
Mitochondrion |
| B. |
Cilium |
II. |
Cell division |
| C. |
Cristae |
III. |
Cell movement |
| D. |
Cell membrane |
IV. |
Phospholipid Bilayer |
Choose the correct answer from the options given below:
| 1. |
A-IV, B-II, C-III, D-I |
2. |
A-II, B-III, C-I, D-IV |
| 3. |
A-I, B-II, C-III, D-IV |
4. |
A-II, B-I, C-IV, D-III |
178. Which one of the following equations represent the Verhulst-Pearl Logistic Growth of population?
| 1. |
\(\frac{d \mathrm{~N}}{d t}=r N\left(\frac{N-K}{N}\right) \) |
2. |
\(\frac{d N}{d t}=N\left(\frac{r-K}{K}\right) \) |
| 3. |
\(\frac{d N}{d t}=r\left(\frac{K-N}{K}\right) \) |
4. |
\(\frac{d N}{d t}=r N\left(\frac{K-N}{K}\right)\) |
179. Match
List-I with
List-II.
|
List-I |
|
List-II |
| A. |
Emphysema |
I. |
Rapid spasms in muscle due to low Ca++ in body fluid |
| B. |
Angina Pectoris |
II. |
Damaged alveolar walls and decreased respiratory surface |
| C. |
Glomerulonephritis |
III. |
Acute chest pain when not enough oxygen is reaching to heart muscle |
| D. |
Tetany |
IV. |
Inflammation of glomeruli of kidney |
Choose the correct answer from the options given below :
1. A-II, B-IV, C-III, D-I
2. A-II, B-III, C-IV, D-I
3. A-III, B-I, C-IV, D-II
4. A-III, B-I, C-II, D-IV
180. Cardiac activities of the heart are regulated by :
| A. |
Nodal tissue |
| B. |
A special neural centre in the medulla oblongata |
| C. |
Adrenal medullary hormones |
| D. |
Adrenal cortical hormones |
Choose the correct answer from the options given below:
| 1. |
A, C and D only |
| 2. |
A, B and D only |
| 3. |
A, B and C only |
| 4. |
A, B C and D |
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course


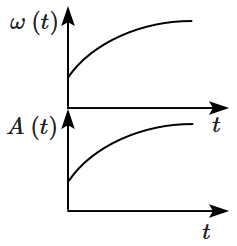















 undergoes \(S_N2\) reaction faster than
undergoes \(S_N2\) reaction faster than