An element R forms the highest oxide R2O5. R belongs to:
1. 13th group
2. 15th group
3. 16 th group
4. 2nd group
An element X is placed in group IA of the periodic table because it forms:
1. An oxide which is acid
2. A volatile chloride having formula XCl2
3. An ionic chloride having formula XCl
4. An insoluble chloride XCl4
Zn and Cd do not show variable valency like 'd' block elements due to :
1. Softness
2. Completed 'd' orbital
3. Two electrons in outermost orbit
4. Low m.p.
The correct arrangement of increasing order of atomic radius among Na, K, Mg and Rb is
1. Mg<K<Na<Rb
2. Mg<Na<K<Rb
3. Mg<Na<Rb<K
4. Na<K<Rb<Mg
In an octahedral structure, the pair of d orbitals involved in d2sp3-hybridisation is:
1.
2.
3.
4.
Equilateral shape has:
1. sp hybridisation
2. sp2 hybridisation
3. sp3 hybridisation
4. dsp2 hybridisation
The coordination number and oxidation state of Cr in K3[Cr(C2O4)3] are respectively
1. 3 and +3
2. 3 and 0
3. 6 and +3
4. 4 and +2
The octahedral complex will not show geometrical isomerism is :
(A and B are monodentate ligands)
1. [MA4B2]
2. [MA5B]
3. [MA2B4]
4. [MA3B3]
Glycinato ligand is :
1. 

2. bidentate ligand
3. two donor sites N and O-
4. all of the above
Among [Ni(CO)4],[Ni(CN)4]2-, [NiCl4]2- species, the hybridisation states of the Ni atom are, respectively (At. no. of Ni=28)
1. sp3,dsp2.dsp2
2. sp3,dsp2,sp3
3. sp3,sp3,dsp2
4. dsp2,sp3,sp3
Which order is correct in spectrochemical series of ligands?
1. Cl-<F-<[C2O4]2-<NO2-<CN-
2. CN-<[C2O4)2-<Cl->NO2-<F-
3. [C2O4)2-<F-<Cl->NO2-<CN-
4. F-<Cl-<NO2-<CN-<[C2O4]2-
The correct order of increasing atomic radius of the following elements is:
1. S < O < Se < C
2. O < C < S < Se
3. O < S < Se < C
4. C < O < S < Se
The species, having bond angles of 120° is :
1. PH3
2. ClF3
3. NCl3
4. BCl3
Which of the following orders of ionic radii is correctly represented?
| 1. | H–>H> | 2. | Na+>F–>O2– |
| 3. | F–>O2–>Na+ | 4. | N3–>Mg2+<Al3+ |
Be2+ is isoelectronic with which of the following ions?
1. H+
2. Li+
3. Na+
4. Mg2+
A molecule having the maximum dipole moment among the following is -
1. CO2
2. CH4
3. NH3
4. NF3
Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour?
1. [Ni(NH3)6]2+
2. [Zn(NH3)6]2+
3. [Cr(NH3)6]3+
4. [Co(NH3)6]3+
The H-O-H bond angles in are approximately . The orbitals used by oxygen in these bond are best describe as:
1.
2. sp hybrid orbitals
3. hybrid orbital
4. hybrid orbital
Which one will show variable oxidation state?
(I) (II)
(III) (IV)
Consider the following conversions:
| (i) | (ii) | ||
| (iii) | (iv) |
That according to given information the incorrect statement is:
| 1. | is more negative than and |
| 2. | is less negative than |
| 3. | , and are negative whereas is positive |
| 4. | and are negative whereas and are positive |
Which of the following order is correct for the property mentioned in brackets?
(Ionisation energy)
(2nd Ionisation energy)
(Electronegativity)
(Ionic radius)
Which species is planar?
(a)
(b)
(c)
(d)
In terms of polar character which of the following order is correct?
1.
2.
3.
4.
Which of the following complex compound(s) is/are paramagnetic and low spin?
(I)
(II)
(III)
(IV)
Choose the correct code :
(1) I only (2) II and III (3) I and IV (4) IV only
An aqueous solution of titanium chloride, when subjected to magnetic measurement, measured to zero magnetic moment. Assuming the octahedral complex in aqueous solution, the formula of the complex is :
1. \(\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2
\)
2. \(\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_4
\)
3. \(\left[\mathrm{TiCl}_3\left(\mathrm{H}_2 \mathrm{O}\right)_3\right]
\)
4. \(\left[\mathrm{TiCl}_2\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]\)
Polarization of electrons in acrolein may be written as:
1.
2.
3.
4.
Which of the following species can act as reducing agent ?
(1)
(2)
(3)
(4)
The following complexes are given :
(1)
(2)
(3)
(4)
(5)
(6)
Choose the correct code:
(a) (1),(2) are optically active, (3) is optically inactive
(b) (2) is optically active ; (1), (3) are optically inactive
(c) (4), (5) are coloured and (6) is colourless
(d) (4) is coloured and (5),(6) are colourless
Which of the following is called Wilkinson's catalyst:
(a) (b)
(c) (d)
Which statement accurately describes an element with an atomic weight of 39 and the electronic configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹?
1. High value of Ionisation Energy
2. Transition element
3. Isotone with \(_{18}^{38}\text{Ar}\)
4. None of the above
There are four elements 'p', 'q', 'r' and 's' having atomic number Z-1, Z, Z+1 and Z+2 respectively. If there element 'q' is an inert gas, select the correct answers from the following statements.
(i) 'p' has most negative electron gain enthalpy in the respective period.
(ii) 'r' is an alkali metal.
(iii) 's' exists in +2 oxidation state.
(1) (i) and (ii)
(2) (ii) and (iii)
(3) (i) and (iii)
(4) (i), (ii) and (iii)
Which of the following orders are correct for the ionization energies?
(A) \( B a < S r < C a \)
(B) \(S^{2 -} < S < S^{2 +}\)
(C) \(C < O < N\)
(D) \(M g < A l < S i\)
1. A, B and D
2. A, C and D
3. A, B and C
4. A, B, C and D
Which of the following are isoelectronic and isostructural ?
(1)
(2)
(3)
(4) All of these
According to Molecular Orbital Theory, which of the following statement is incorrect?
1. C2 has no unpaired electrons.
2. In molecule, both the bonds are bonds.
3. is paramagnetic but is diamagnetic.
4. In ion, there is one and two bonds.
When two atoms combine to form a molecule, energy is:
| 1. | Released. | 2. | Absorbed. |
| 3. | Neither released nor absorbed. | 4. | Either released or absorbed. |
Valency is best described as:
1. Total electrons in an atom
2. Atomicity of an element
3. Oxidation number of an element
4. Combining capacity of an element
Which one of the following ions is the most stable in an aqueous solution?
(At. No. Ti = 22, V = 23, Cr = 24, Mn = 25)
1. \(\text{Cr}^{3 +}\)
2. \(\text V^{3 +}\)
3. \(\text{Ti}^{3 +}\)
4. \(\text{Mn}^{3 +}\)
Assuming that elements are formed to complete the seventh period, what would be the atomic number of alkaline earth metal of the eighth period?
1. 113
2. 120
3. 119
4. 106
Which of the following molecules or ions is not linear ?
1. BeCl2
2.
3. CS2
4.
Lead poisoning in the body can be removed by:
1. EDTA in the form of calcium dihydrogen salt
2. cis-platin
3. Zeisse’s salt
4. DMG
The numbers of lone pairs and bond pairs in hydrazine are, respectively
1. 2 and 4
2. 2 and 6
3. 2 and 5
4. 1 and 5
The number of hydrogen bonded water molecules(s) associated with \(\mathrm{C u S O_{4} . 5 H_{2} O}\) is:
| 1. | 3 | 2. | 1 |
| 3. | 2 | 4. | 5 |
Which of the following statements is not correct?
1. The complexes and differ in the state of hybridization of iron
2. The complexes and differ in the magnetic properties
3. iron ion has the same secondary valency in the complexes and
4. The complex and differ in primary valency of iron
Among i. [Co(NH3)6]CI3 , ii. [Ni(NH3)6]CI2 , iii. Cr(H2O)6]CI3 , iv. [Fe(H2O)6]CI2, the diamagnetic complex is-
1. i
2. ii
3. iii
4. iv
The Crystal Field Stabilization Energy (CFSE) and the spin-only magnetic moment in Bohr Magneton (BM) for the complex are, respectively,
1.
2.
3.
4.
The correct electronic configuration for the complex [Ni(NH3)6]2+ is:
1.
2.
3.
4.
Among
(i) [Cr(en)3]3+
(ii) trans-[Cr(en)2Cl2]+
(iii) cis-[Cr(en)2Cl2]+
(iv) [Co(NH3)4Cl2]+
the optically active complexes are-
1. (i), (ii) and (iii)
2. (i) and (iii)
3. (ii) and (iii)
4. (ii) and (iv)
How many isomers are possible for complex [Co(Ox)2Cl2]3-?
1. 1
2. 3
3. 2
4. 4
The increasing order of oxidising power among the following species is:
1. \(\mathrm{VO}_2^{+}<\mathrm{Cr}_2 \mathrm{O}_7^{2-}<\mathrm{MnO}_4^{-}\)
2. \(\mathrm{VO}_2^{+}<\mathrm{MnO}_4^{-}<\mathrm{Cr}_2 \mathrm{O}_7^{2-}\)
3. \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}<\mathrm{VO}_2^{+}<\mathrm{MnO}_4^{-}\)
4. \(\mathrm{Cr}_2 \mathrm{O}_7{ }^{2-}<\mathrm{MnO}_4^{-}<\mathrm{VO}_2^{+}\)
The number of (i) sp2 hybridized carbon atoms and (ii) bonds are present in the following compound are: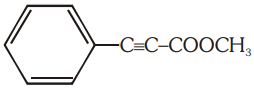
1. 7, 5
2. 8, 6
3. 7, 6
4. 8, 5