What is the ratio of the time period of revolution of electron He+ ion sample in the orbit where the path length is three time the de-broglies wavelength to the time period of revolution in the ground state –
1. 27:1
2. 1:27
3. 9:8
4. 8:9
In the reaction;
2Ag + 2H2SO4 Ag2SO4 + 2H2O + SO2, H2SO4 acts as:
1. Oxidizing agent
2. Reducing agent
3. Dehydrating agent
4. None of the above
Select the intermediate in the following reaction mechanism:
O3(g) O2(g) +O(g)
O(g) +O3(g) 2O2(g)
1. O3(g)
2. O(g)
3. O2(g)
4. none of these
Incorrectly matched characteristic is:
1. S8: covalent lattice
2. P4: tetrahedron
3. S52-: zigzag
4. SiO2: Covalent lattice
Which of the following cation does not form an amine complex with excess of ammonia?
1. Ag+
2. Cu2+
3. Cd2+
4. Na+
The reaction, RCl +NaI + NaCl is known as:
1. Wurtz reaction
2. Fittig reaction
3. Frankland's reaction
4. Finkelstein's reaction
The correct order of second ionisation energies of B, C, N, & O is
1. O > N > C > B
2. O > C > N > B
3. O > B > C > N
4. O > N >B > C
Which is mismatched regarding the shape ?
(1) XeF4 = Square planar
(2) XeOF4 = Square Pyramidal
(3) XeF6 = Distorted Octahedral
(4)XeO3 = Bent Shape
Actinide contraction is
mainly related to:
1. Poor shielding by 5f subshell
2. Extraordinary decrement in Zeff
3. Both (1) and (2)
4. Strong shielding by 5f subshel
What will be the value of for the rection
if
(1) -65.62 KJ
(2) -75.27 KJ
(3) -150.54 KJ
(4) +150.54 KJ
The IUPAC name of 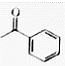 is
is
(1) 2-Phenyl ethanone
(2) 1-phenyl ethanone
(3) 1- (Oxoethyl) benzene
(4) 1 - (Ethyaloxo) benzene
Which substrate is appropriate for the preparation of methane and ethane in a single-step process?
1. H2C = CH2
2. CH3OH
3. CH3-Br
4. CH3-CH2-OH
Above compounds (P) & (Q) can be differentiated by:
(1) amm. AgNO3
(2) NaOH
(3) FeCl3
(4) Both (a) & (b)
Give test to differentiate 1,1-dichloroethane and 1, 2-dichloroethane:
1. 2,4-DNP then aq. KOH
2. Aq. KOH then 2, 4-DNP
3. NaHSO3
4. Lucas reagent
Reagent not used to prepare an alkyl halide from an alcohol is:
(1) HCl+ ZnCl2
(2) NaCl
(3) PCl5
(4) SOCl2
Diacetone alcohol is obtained by the reaction of:
(1) acetone and ethanol
(2) acetone and conc. H2SO4
(3) acetone and Ba(OH)2
(4) acetone and Al(OH)3
Keratin, a structural protein is present in:
1. hair
2. skin
3. wool
4. All of the above
A vitamin which plays a vital role in the coagulating property of blood is:-
(1) vitamin A
(2) vitamin D
(3) vitamin B
(4) vitamin K
The process of respiration in absence of oxygen is called:
(1) metabolic
(2) aerobic
(3) anaerobic
(4) glycolysis
Acetylene can be converted to higher alkyne using the following sequence of reactions:
(1) Na,RX
(2) RMg X,R X
(3) either of these two
(4) none of these
The formation of cyanohydrin from a ketone is an example of:
(1) electrophilic addition
(2) nucleophilic addition
(3) nucleophilic substitution
(4) electrophilic substitution
Boric acid is an acid because its molecule
(1) contains replaceable H+ ion
(2) gives up a proton
(3) accepts OH- from water releasing proton
(4) combines with proton from water molecule
consider the following compounds
hyperconjugation occurs in
1. I only
2. II only
3. III only
4. I and III
The name of complex ion, [Fe(CN)6]3- is :-
1. hexacyanoiron (III) ion
2. hexacyanitoferrate (III) ion
3. tricyanoferrate (III) ion
4. hexacyanidoferrate (III)ion
What is the molality of the solution of a certain solute in a solvent if there is a freezing point depression of 0.184° and if the freezing point constant is 18.4 K kg mol−1
1. 0.01
2. 1.00
3. 0.001
4. 100
The amount of ice that will seprate out on cooling a solution containing 50 g of ethylene
glycol in 200 g water to -9.3 C is: (
1. 38.71 g
2. 38.71 mg
3. 42 g
4. 42 mg
An irreversible process occuring isothermally in an isolated system leads to
(1) Zero entropy
(2) An increase in the total entropy of the system
(3) A decrease in the total entropy of the system
(4) None of these
The gas that cannot be collected over water is:
1.
2.
3.
4.
In which case the racemic mixture is obtained by mixing its mirror images (d & l form) in 1:1 molar ratio?
1. [Co(gly)3]
2. [Ni(DMG)2]
3. cis-[Cu(gly)2]
4. [Zn(en)(gly)]+
The electric conduction of a salt solution in water depends on the:
1. Size of its molecules
2. Shape of its molecules
3. Size of solvent molecules
4. Extent of its ionization
For a reaction of the type 2A+B 2C, the rate of the reaction is given by . When the volume of the reaction vessel is reduced to 1/4 th of the original volume, the rate of reaction changes by a factor of
(A) 0.25
(B) 16
(C) 64
(D) 4
The heat of formation of HCl(g) from the reaction
H2(g) + Cl2(g) ; is
1. +44 kcal
2. -44 kcal
3. +22 kcal
4. -22 kcal
Which of the following has maximum boiling point-
(1)
(2)
(3)
(4)
According to Bohr’s theory the angular momentum of an electron in the fourth orbit is
1.
2.
3.
4.
A 6.85 g sample of the hydrates is dried in an oven to give 3.13 g of anhydrous . The value of x is -
(Atomic weights : Sr=87.60, O=16.0, H=1.0)
1. 8
2. 12
3. 10
4. 6
Density of dry air containing only and is 1.15 g/L at 740 mm and 300 K. What is % composition of by weight in the air?
1. 78%
2. 75.5%
3. 80.5%
4. 72.75%
Which among the given molecules can exhibit tautomerism?
1. III only
2. Both I and III
3. Both I and II
4. Both II and III
Benzaldehyde cannot be prepared by:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Three-dimensional molecules with cross-links are formed in case of a :
1. Thermoplastic
2. Thermosetting plastics
3. Both 1 and 2
4. None of these
The first four I.E. values of an element are 284, 412, 656 and 3210 kJ mol-1. The number of valence electrons in the element are :
1. one
2. two
3. three
4. four
The half cell reduction potential of a hydrogen electrode at pH = 10 will be :
1. 0.59 V
2. – 0.59 V
3. 0.059 V
4. – 0.059 V
Anhydrous aluminium chloride fumes in moist air owing to the formation of :
1. gaseous aluminium chloride
2. chlorine
3. chlorine dioxide
4. hydrogen chloride
Which of the following is a redox reaction?
1. with NaOH
2. Formation of in the atmosphere
3. Evaporation of
4. Oxides of nitrogen forming nitrogen & oxygen from lightening
Silica is reacted with sodium carbonate. Which is the gas liberated?
(1) CO
(2)
(3)
(4)
What is the final product formed when glycerol reacts with oxalic acid at 260°C?
1. Formic acid
2. Allyl alcohol
3. Acrolein
4. Acetic Acid
The correct order of E0(M2+/M) values with a negative sign for the four successive elements Cr, Mn, Fe and Co is
1. Mn > Fe > Co > Cr
2. Mn > Cr > Fe > Co
3. Fe > Mn > Cr > Co
4. Co > Fe > Mn > Cr
Aqueous solution of urea is:
1. Acidic
2. Alkaline
3. Almost neutral
4. Amphoteric
Determine the value of equilibrium constant (Kc) for the reaction
If 10 moles of A2; 15 moles of B2 and 5 moles of AB are placed in a 2 litre vessel and allowed to come to equilibrium. The final concentration of AB is 7.5 M:
1. 4.5
2. 1.5
3. 0.6
4. None of these
Find the molar solubility of Fe(OH)3 in a buffer solution that is 0.10 M in NH4Cl and 0.10 M in NH3 :
\(\mathrm{K}_{\mathrm{b}}\left(\mathrm{NH}_3\right)=1.8 \times 10^{-5}\) and
\(\text K_{ \text {sp}} [ \text {Fe(OH)}_3] = 2.6 \times 10^{-39}\)
| 1. | 4.458 × 10–25 M | 2. | 3.458 × 10–25 M |
| 3. | 2.229 × 10–24 M | 4. | 4.458 × 10–22 M |
Which of the following amines doesn't give the carbylamine reaction?
| 1. | Ethylamine | 2. | Dimethylamine |
| 3. | Methylamine | 4. | Phenylamine |