The correct IUPAC name of the given compound is:
1. 2-Chloro-5-hydroxyhexane
2. 2-Hydroxy-5-chlorohexane
3. 5-Chlorohexan-2-ol
4. 2-Chlorohexan-5-ol
Which of the following reagents can be used to oxidize primary alcohols to aldehydes?
I. in anhydrous medium
II. in acidic medium
III. Pyridinium chlorochromate
IV. Heat in the presence of Cu at 573K
Choose the correct option:
1. (I, II, III)
2. (II, III, IV)
3. (I, III, IV)
4. (I, II, IV)
In the above reaction, the structure of ‘A’ and the type of isomerism shown in the final product are, respectively,:
| 1. | Prop-1-en-2-ol and Metamerism |
| 2. | Prop-1-en-1-ol and Tautomerism |
| 3. | Prop-2-en-2-ol and Geometrical isomerism |
| 4. | Prop-1-en-2-ol and Tautomerism |
The reagent which does not react with both, acetone and benzaldehyde?
1. Sodium hydrogen sulphite
2. Phenyl hydrazine
3. Fehling's solution
4. Grignard reagent
Through which of the following reactions number of carbon atoms can be increased in the chain?
a. Grignard reaction
b. Cannizzaro’s reaction
c. Aldol condensation
d. HVZ reaction
Choose the correct option:
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
The compound(s) among the following that is/are not typically synthesized using the Sandmeyer reaction:
(a) Chlorobenzene
(b) Bromobenzene
(c) Iodobenzene
(d) Fluorobenzene
Choose the correct option:
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
Select the correct option based on statements below:
| Assertion (A): | Only a small amount of HCI is required in the reduction of nitro compounds with iron scrap and HCI in the presence of steam. |
| Reason (R): | FeCl2 formed gets hydrolyzed to release HCI during the reaction. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Each polypeptide in a protein has amino acids linked with each other in a specific sequence. This sequence of amino acids is said to be:
1. Primary structure of proteins
2. Secondary structure of proteins
3. Tertiary structure of proteins
4. Quaternary structure of proteins
Given below are two statements:
| Assertion (A): | In presence of an enzyme, the substrate molecule can be attacked by the reagent effectively. |
| Reason (R): | Active sites of enzymes hold the substrate molecule in a suitable position. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are FALSE. |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
The hybridization of nitrogen in and respectively is :
1. sp, sp3, and sp2
2. sp, sp2, and sp3
3. sp2, sp, and sp3
4. sp2, sp3, and sp
Which of the following attain the linear structure?
(a)
(b)
(c)
(d)
Choose the correct option
| 1. | (a), (d) | 2. | (b), (c) |
| 3. | (c), (d) | 4. | (b), (d) |
If the electronic configuration of an element is , the four electrons that participate in the chemical bond formation will be from :
| 1. | 2. | ||
| 3. | 4. |
Given below are two statements:
| Assertion (A): | Sodium chloride, formed by the action of chlorine gas on sodium metal, is a stable compound. |
| Reason (R): | Sodium and chloride ions acquire octets in sodium chloride formation. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Which of the following statements is not correct about the order of a reaction?
| 1. | The order of a reaction can be a fractional number |
| 2. | Order of a reaction is experimentally determined quantity |
| 3. | The order of a reaction is always equal to the sum of the stoichiometric coefficients of reactants in the balanced chemical equation for a reaction |
| 4. | The order of a reaction is the sum of the powers of the molar concentration of the reactants in the rate law expression |
Consider the reaction AB. The concentration of both the reactant and the product varies exponentially with time.
The graph that accurately depicts how reactant and product concentrations change with time is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The ionic radii vary in :
| (a) | Inverse proportion to the effective nuclear charge. |
| (b) | Inverse proportion to the square of effective nuclear charge. |
| (c) | Direct proportion to the screening effect. |
| (d) | Direct proportion to the square of screening effect. |
Choose the correct option :
1. (a), (c)
2. (b), (c)
3. (c), (d)
4. (b), (d)
The elements with atomic numbers 35, 53, and 85 are:
| 1. | Noble gases | 2. | Halogens |
| 3. | Heavy metals | 4. | Light metals |
Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is:
| 1. | F > Cl > Br > I | 2. | F < Cl < Br < I |
| 3. | F < Cl > Br > I | 4. | F < Cl < Br > I |
Amongst the following elements whose electronic configuration are given below, the one having the highest ionisation enthalpy is:
| 1. | 2. | ||
| 3. | 4. |
Given below are two statements:
| Assertion (A): | The increasing order or acidity of hydrogen halides is HF<HCI<HBr<HI. |
| Reason (R): | While comparing acids formed by the elements belonging to the same group of the periodic table, H-A bond strength is a more important factor in determining the acidity of an acid than the polar nature of the bond. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
for is and Kb for is . The pH of ammonium acetate will be:
1. 7.005
2. 4.75
3. 7.0
4. Between 6 and 7
are the respective ionisation constants for the following reactions.
\(\mathrm{H}_2 \mathrm{~S} \rightleftharpoons \mathrm{H}^{+}+\mathrm{HS}^{-}\)
\(\mathrm{HS}^{-} \rightleftharpoons \mathrm{H}^{+}+\mathrm{S}^{2-}\)
\(\mathrm{H}_2 \mathrm{~S} \rightleftharpoons 2 \mathrm{H}^{+}+\mathrm{S}^{2-}\)
The correct relationship between is:
1. \(\mathrm{K}_{\mathrm{a}_3}=\mathrm{K}_{\mathrm{a}_1} \times \mathrm{K}_{\mathrm{a}_2} \)
2. \(\mathrm{K}_{\mathrm{a}_3}=\mathrm{K}_{\mathrm{a}_1}+\mathrm{K}_{\mathrm{a}_2} \)
3. \(K_{a_3}=K_{a_1}-K_{a_2} \)
4. \(\mathrm{K}_{\mathrm{a}_3}=\mathrm{K}_{\mathrm{a}_1} / \mathrm{K}_{\mathrm{a}_2}\)
The compounds [Co(SO (NH) ]Br and [Co(SO (NH)] Cl classified as:
| 1. | Linkage isomerism | 2. | Ionisation isomerism |
| 3. | Coordination isomerism | 4. | No isomerism |
An aqueous pink solution of cobalt(II) chloride changes to deep blue on the addition of excess of HCl. This is because:
| a. | \([Co(H_2O)_6]^{2+}\) is transformed into \([CoCl_6]^{4-}\) |
| b. | \([Co(H_2O)_6]^{2+}\) is transformed into \([CoCl_4]^{2-}\) |
| c. | Tetrahedral complexes have smaller crystal field splitting than octahedral complexes |
| d. | Tetrahedral complexes have larger crystal field splitting than octahedral complexes |
Choose the correct option:
1. a and b
2. b and c
3. c and d
4. a and c
| Assertion (A): | and are reducing in nature. |
| Reason (R): | Unpaired electrons are present in their d-orbitals. |
| 1. | Both (A) and (R) are True, and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True, and (R) is not the correct explanation of (A). |
| 3. | (A) is True, but the (R) is False. |
| 4. | (A) is False, but (R) is True. |
Match the complex ions give in Column I with the colours given in column II and assign the correct code.
|
Column I (Complex ion) |
Column II (Colour) |
|
A. |
1. Violet |
|
B. |
2. Green |
|
C. |
3. Pale blue |
|
D. |
4. Yellowish orange |
Codes:
| Options: | A | B | C | D |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 5 | 2 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 3 | 2 | 1 |
Use the data given above to find out the most stable ion in its reduced form.
| 1. | Cl- | 2. | Cr3+ |
| 3. | Cr | 4. | Mn2+ |
Using the data given below find out the strongest oxidizing agent.
\(\mathrm{E_{Cr_{2} O_{7}^{2 -} / Cr^{3 +}}^{\ominus} = 1 . 33 V ; E_{Cl_{2} / Cl^{-}}^{\ominus} = 1 . 36 V \\ E_{Mn O_{4}^{-} / Mn^{2 +}}^{\ominus} = 1 . 51 V ; E_{Cr^{3 +} / Cr}^{\ominus} = - 0 . 74 V}\)
Transition elements form binary compounds with halogens. Which of the following elements will form MF3 type compounds?
| (a) | Cr | (b) | Co |
| (c) | Cu | (d) | Ni |
Choose the correct option
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |
acts as an oxidizing agent in an acidic medium. The number of moles of that will be needed to react with one mole of sulphide ions in an acidic solution is
| 1. | \(\dfrac{2}{5}\) | 2. | \(\dfrac{3}{5}\) |
| 3. | \(\dfrac{4}{5}\) | 4. | \(\dfrac{1}{5}\) |
There are 14 elements in the actinoid series. Which of the following elements does not belong to this series?
| 1. | U | 2. | Np |
| 3. | Tm | 4. | Fm |
In the listed lanthanoids, the element that displays a +2 oxidation state along with the characteristic +3 oxidation state of lanthanoids is:
(a) Ce
(b) Eu
(c) Yb
(d) Ho
Choose the correct option
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of . Which of the following species attacks the benzene ring in this reaction?
Consider the given two statements:
| Assertion (A): | KCN reacts with methyl chloride to give methyl isocyanide. |
| Reason (R): | \(CN^-\) is an ambident nucleophile. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
| Assertion (A): | NaCl reacts with concentrated H2SO4 to give colourless fumes with a pungent smell. But on adding MnO2, the fumes become greenish-yellow. |
| Reason (R): | MnO2 oxidizes HCl to chlorine gas which is greenish-yellow. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
A compound X, of boron, reacts with NH3 on heating to give another compound Y which is called inorganic benzene. The compound X can be prepared by treating BF3 with lithium aluminum hydride. The compounds X and Y are represented by the formulas.
1. B2H6, B3N3H6
2. B2O3, B3N3H6
3. BF3, B3N3H6
4. B3N3H6, B2H6
Arrange the following carbanions in order of their decreasing stability:
A. \(\mathrm{H}_3 \mathrm{C}-\mathrm{C} \equiv \mathrm{C}^{-}\)
B. \(\mathrm{H}-\mathrm{C} \equiv \mathrm{C}^{-}\)
C. \(\mathrm{H}_3 \mathrm{C}-\mathrm{{C}H_2^-}\)
| 1. | A > B > C | 2. | B > A > C |
| 3. | C > B > A | 4. | C > A > B |
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring:
| A | Deactivates the ring by inductive effect |
| B | Deactivates the ring by resonance |
| C | Increases the charge density at the ortho and the para positions relative to the meta position by resonance. |
| D | Directs the incoming electrophile to the meta position by increasing the charge density relative to the ortho and the para positions. |
Choose the correct option:
| 1. | A and B | 2. | B and C |
| 3. | C and D | 4. | A and C |
Consider the following four compounds.
| I. | 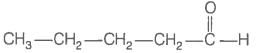 |
| II. | 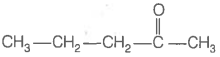 |
| III. | 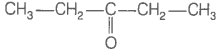 |
| IV. | 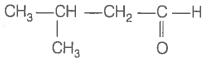 |
Consider the following pairs of compounds.
(a) II and III
(b) II and IV
(c) I and IV
(d) I and II
Choose the pairs given above that are not functional group isomers:
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Paper chromatography operates based on which of the following principles?
1. Adsorption
2. Partition
3. Solubility
4. Volatility
| 1. | Increases with an increase in temperature. |
| 2. | Decreases with an increase in temperature. |
| 3. | Remains constant. |
| 4. | First increases then decreases. |
In the reactions given below, thiosulphate reacts differently with iodine than with bromine.
Choose the statements among the following that best describe the above dual behaviour of thiosulphate.
| 1. | Bromine is a stronger oxidant than iodine. |
| 2. | Bromine is a weaker oxidant than iodine. |
| 3. | Thiosulphate undergoes oxidation by bromine and reduction by iodine in these reactions. |
| 4. | Bromine undergoes oxidation and iodine undergoes reduction in these reactions. |
The maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon:
| 1. | Temperature | 2. | Nature of solute |
| 3. | Pressure | 4. | Nature of solvent |
One mole of oxygen gas at STP is equal to:
| (a) | 6 .022× 1023 molecules of oxygen |
| (b) | 6.022 × 1023 atoms of oxygen |
| (c) | 16 g of oxygen molecule |
| (d) | 32 g of oxygen |
Choose the correct answer from the option given below:
1. (a) and (b)Out of the following pairs of electrons, identify the pairs of electrons present in degenerate orbitals.
| (a) | (i) n = 3, l = 2, ml = –2, ms = –\(\frac{1}{2}\) (ii) n = 3, l = 2, ml = –1, ms = –\(\frac{1}{2}\) |
| (b) | (i) n = 3, l = 1, ml = +1, ms = +\(\frac{1}{2}\) (ii) n = 3, l = 2, ml = +1, ms = +\(\frac{1}{2}\) |
| (c) | (i) n = 4, l = 1, ml = +1, ms = +\(\frac{1}{2}\) (ii) n = 3, l = 2, ml = +1, ms = +\(\frac{1}{2}\) |
| (d) | (i) n = 3, l = 2, ml = +2, ms = –\(\frac{1}{2}\) (ii) n = 3, l = 2, ml = +2, ms = +\(\frac{1}{2}\) |
1. (a), (d)
2. (b), (c)
3. (c), (d)
4. (b), (d)
The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ______.
1.
2.
3.
4.
| Assertion (A): | The empirical mass of ethene is half of its molecular mass. |
| Reason (R): | The empirical formula represents the simplest whole-number ratio of the various atoms present in a compound. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
In an adiabatic process, no transfer of heat takes place between the system and its surroundings. The correct option for free expansion of an ideal gas under adiabatic condition from the following is:
1.
2.
3.
4.
| (a) | \(dU = 0 \) | (b) | \(dQ = 0\) |
| (c) | \(dQ = dU \) | (d) | \(dQ = dW\) |
| 1. | (a), (b), (c) | 2. | (a), (d) |
| 3. | (b), (c), (d) | 4. | (a), (c), (d) |