Botany - Section A
1. Endosperm is formed during double fertilization by
| 1. |
two polar nuclei and one male gamete |
| 2. |
one polar nuclei and one male gamete |
| 3. |
ovum and male gametes |
| 4. |
two polar nuclei and two male gametes |
2. How many pairs of contrasting traits in pea plants were studied by Mendel in his experiments?
1. Five
2. Six
3. Eight
4. Seven
3. Which one of the following pairs of nitrogenous bases of nucleic acids is wrongly matched with the category mentioned against it?
1. Thymine, Uracil - Pyrimidines
2. Uracil, Cytosine - Pyrimidines
3. Guanine, Adenine - Purines
4. Adenine, Thymine - Purines
4. "The activated sludge is pumped into large tanks. Here, other kinds of bacteria, which grow anaerobically, digest the bacteria and the fungi in the sludge". In which tank, this process of sewage treatment is performed?
1. Secondary settling tank
2. Aeration tank
3. Primark settling tank
4. Anaerobic sludge digesters
5. Similar biological structures or sequences in different taxa are homologous if they:
| 1. |
always perform the same function |
| 2. |
result from convergent evolution |
| 3. |
do not serve any known function |
| 4. |
are derived from a common ancestor |
6. If a bamboo plant is growing in a far forest then what will be the trophic level of it?
1. First trophic level (T1)
2. Second trophic level (T2)
3. Third trophic level (T3)
4. Fourth trophic level (T4)
7. The floral formula has which of the following symbols to represent zygomorphic symmetry?
1. #
2. %
3. K
4. C
8. Mode of nutrition in euglenoids is:
1. Photosynthetic
2. Heterotrophic
3. Chemosynthetic
4. Both 1 & 2
9. Which one is/are correct?
| 1. |
In prokaryotes, a single type of RNA polymerase can transcribe mRNA, tRNA, and rRNA. |
| 2. |
In eukaryotes, RNA polymerase I transcribe rRNAs (28S, 18S, and 5.8S), whereas RNA polymerase III is responsible for the transcription of tRNA, 5srRNA, and snRNAs. |
| 3. |
RNA pol II transcribes hnRNA in eukaryotes. |
| 4. |
Ribosomal large subunit has p and A-sites. |
| 1. |
A, C |
| 2. |
A, B, C, D |
| 3. |
B, C, D |
| 4. |
A, B, D |
10. Frameshift mutation arises due to:
| 1. |
Addition or deletion of a nucleotide base in DNA |
| 2. |
Loss and gain of DNA segments respectively |
| 3. |
Shifting of a part of chromosome |
| 4. |
Rotating a section of the chromosome by 180° |
11. What is the fate of the male gametes discharged in the synergid?
| 1. |
One fuses with the egg and the other fuses with central cell nuclei |
| 2. |
One fuses with the egg and other(s) degenerate(s) in the synergid |
| 3. |
All fuse with the egg |
| 4. |
One fuses with the egg and the other(s) fuse(s) with the synergid nucleus |
12. Which of the following is a ribozyme in bacteria for the formation of peptide bonds?
1. 28 S rRNA
2. 5.8 S rRNA
3. 23 S rRNA
4. 16 S rRNA
13.
| Assertion (A): |
Ribosomal RNA is synthesized in the nucleus of the cell. |
| Reason (R): |
It is translated with the enzyme RNA polymerase III. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
14. In a certain species of insects, some have 13 chromosomes, and the others have 14 chromosomes. The 13 and 14 chromosome bearing organisms are
| 1. |
males and females, respectively |
| 2. |
females and males, respectively |
| 3. |
all males |
| 4. |
all females |
15. The enzyme decarboxylase catalyses which of the following step:
| 1. |
conversion of citric acid to cis aconitic acid |
| 2. |
fumaric acid to malic acid |
| 3. |
oxalosuccinic acid to α -ketoglutaric acid |
| 4. |
malic acid to oxaloacetic acid |
16. How does pruning help in making the hedge dense?
| 1. |
It induces the differentiation of new shoots from the rootstock |
| 2. |
It frees axillary buds from apical dominance |
| 3. |
The apical shoot grows faster after pruning |
| 4. |
It releases wound hormones |
17. Sacred groves are especially useful in:
| 1. |
Generating environmental awareness |
| 2. |
Preventing soil erosion |
| 3. |
Year-round flow of water in rivers |
| 4. |
Conserving rare and threatened species |
18. Find the incorrect match.
1. Triticum aestivum: Caryopsis
2. Feathery stigma: Cereals
3. Sugarcane: Compositae family
4. Unisexual flowers: Zea mays
19. Pentamerous actinomorphic flowers, bicarpellary ovary with an oblique septum, and fruit of capsule or berry belong to which of the following family?
1. Liliaceae
2. Asteraceae
3. Brassicaceae
4. Solanaceae
20. The primitive prokaryotes responsible for the production of biogas from the dung of ruminant animals include:
1. Eubacteria
2. Halophiles
3. Thermoacidophiles
4. Methanogens
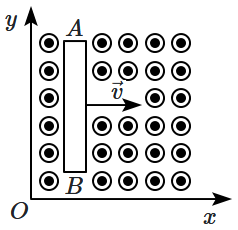
21. Identify the correct match:
1. Felis: Canidae
2. Datura: Solanaceae
3. Petunia: Leguminoceae
4. Dogs: Felidae
22. Which of the following nucleic acids is present in an organism having 70 S ribosomes only?
| 1. |
Single stranded DNA with a protein coat |
| 2. |
Double stranded circular naked DNA |
| 3. |
Double stranded DNA enclosed in a nuclear membrane |
| 4. |
Double stranded circular DNA with histone proteins |
23. One scientist cultured Cladophora in a suspension of Azotobacter and illuminated the culture by splitting light through a prism. He observed that bacteria accumulated mainly in the region of :
1. Violet and green light
2. Indigo and green light
3. Orange and yellow light
4. Blue and red light
24. What type of pollination takes place in Vallisneria?
| 1. |
Pollination occurs in submerged conditions by water. |
| 2. |
Flowers emerge above the surface of the water and pollination occurs by insects. |
| 3. |
Flowers emerge above the water's surface and pollen is carried by the wind. |
| 4. |
Male flowers are carried by water currents to female flowers at the surface of the water. |
25. Grass leaves curl inwards during very dry weather. Select the most appropriate reason from the following :
| 1. |
Tyloses in vessels |
| 2. |
closure of stomata |
| 3. |
Flaccidity of bulliform cells |
| 4. |
Shrinkage of air spaces in spongy mesophyll |
26. Match the following columns and select the correct option.
| Column I |
Column II |
| (a) |
Bt cotton |
(i) |
Gene therapy |
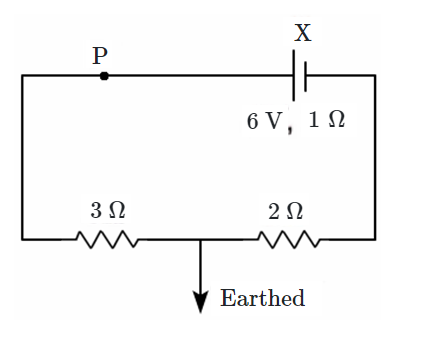
| (b) |
Adenosine deaminase deficiency |
(ii) |
Cellular defence |
| (c) |
RNAi |
(iii) |
Detection of HIV infection |
| (d) |
PCR |
(iv) |
Bacillus thuringiensis |
Options:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(iii) |
(ii) |
(i) |
(iv) |
| 2. |
(ii) |
(iii) |
(iv) |
(i) |
| 3. |
(i) |
(ii) |
(iii) |
(iv) |
| 4. |
(iv) |
(i) |
(ii) |
(iii) |
27. Match the following techniques or instruments with their usage:
| Column I |
Column II |
| (a) |
Bioreactor |
(i) |
Separation of DNA fragments |
| (b) |
Electrophoresis |
(ii) |
Production of large quantities of products |
| (c) |
PCR |
(iii) |
Detection of a pathogen, based on antigen-antibody reaction |
| (d) |
ELISA |
(iv) |
Amplification of nucleic acids |
Select the correct option from the following:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(iii) |
(ii) |
(iv) |
(i) |
| 2. |
(ii) |
(i) |
(iv) |
(iii) |
| 3. |
(iv) |
(iii) |
(ii) |
(i) |
| 4. |
(ii) |
(i) |
(iii) |
(iv) |
28. Which of the following statements is incorrect?
| 1. |
Biomass decreases from the first to fourth trophic level |
| 2. |
Energy content gradually increases from the first to fourth trophic level |
| 3. |
Number of individuals decreases from the first trophic level to the fourth trophic level |
| 4. |
Energy content gradually decreases from the first to the fourth trophic level |
29.
| Assertion (A): |
Tapetum is formed during the process of the formation of microsporangium. |
| Reason (R): |
It plays an important role in guiding the pollen tube into the synergid. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
30. Match the following columns and select the correct option:
| Column - I |
Column -II |
| a. |
Dragonflies |
(i) |
Biocontrol agents of several plant pathogens |
| b. |
Bacillus thuringiensis |
(ii) |
Get rid of mosquitoes |
| c. |
Glomus |
(iii) |
Narrow-spectrum insecticidal applications |
| d. |
Baculoviruses |
(iv) |
Biocontrol agents of lepidopteran plant pests |
|
|
(v) |
Absorb phosphorus from soil |
Options:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(iii) |
(v) |
(iv) |
(i) |
| 2. |
(ii) |
(i) |
(iii) |
(iv) |
| 3. |
(ii) |
(iii) |
(iv) |
(v) |
| 4. |
(ii) |
(iv) |
(v) |
(iii) |
31. Genotypic ratio of 1:2:1 is obtained in a cross between:
1. AA X AA
2. Aa X Aa
3. Aa X aa
4. aa X aa
32. Which of the following algae produces Carrageen?
1. Red algae
2. Blue-green algae
3. Green algae
4. Brown algae
33. In the exponential growth equation Nt =N0ert, e represents:
1. The base of natural logarithms
2. The base of geometric logarithms
3. The base of number logarithms
4. The base of exponential logarithms
34. Which is the "only enzyme" that has the "capability to catalyze initiation, elongation, and termination" in the process of transcription in prokaryotes?
1. DNA Ligase
2. DNase
3. DNA dependent DNA polymerase
4. DNA dependent RNA polymerase
35. When lactic acid bacteria (LAB) is used for converting milk to curd, it improves the nutritional quality of curd by increasing:
1. Iron content of the curd
2. Vitamin B12 content of the curd
3. Vitamin D content of the curd
4. Protein content of the curd
Botany - Section B
36. The given diagram shows the transverse section of a portion of a:

| 1. |
Monocot stem |
2. |
Monocot root |
| 3. |
Dicot stem |
4. |
Dicot root |
37. Identify the labels A, B, C, and D in the figure given below:

|
A |
B |
C |
D |
| 1. |
endosperm |
scutellum |
plumule |
seed coat |
| 2. |
aleurone |
endosperm |
radicle |
coleorhiza |
| 3. |
epithelium |
aleurone |
plumule |
seed coat |
| 4. |
endosperm |
aleurone |
plumule |
seed coat and fruit wall |
38. Correct statement in relation to vacuoles is:
| 1. |
It is a triple membrane-bound space found in cytoplasm containing sap. |
| 2. |
It can occupy 90% of cell volume in plants. |
| 3. |
Its membrane allows the transport of materials along the concentration gradient only. |
| 4. |
Concentration of ions is significantly lesser in vacuole than in cytoplasm. |
39. Find the odd one with respect to the fungi imperfecti:
1. Alternaria
2. Colletotrichum
3. Trichoderma
4. Penicillium
40. The edible part of the mango is:-
1. Receptacle
2. Epicarp
3. Mesocarp
4. Endocarp
41. Select the odd one with respect to developmental plasticity:
1. Buttercup
2. Cotton
3. Larkspur
4. Coriander
42. Which of the following does not apply to the Poaceae family?
1. Annual and perennial herbs and shrubs
2. Bamboo tree
3. Adventitious and fibrous roots
4. Almost 1000 genera
43. A male insect mistakes an orchid flower to be female due to its shape and performs the act of copulation and induces pollination. This is an example of:
1. Mimicry
2. Pseudocopulation
3. Pseudo pollination
4. None
44. Find the odd one concerning the disc florets.
1. Zygomorphic symmetry
2. 5 petals
3. Colored corolla
4. Pappus
45. Remnants of nucellus are persistent during seed development in:
| 1. |
pea |
2. |
black pepper |
| 3. |
wheat |
4. |
groundnut |
46. Match the following:
| Algae |
Stored food |
Flagellation |
| (a) Brown algae |
(p) Floridean starch |
(i) 2, lateral |
| (b) Red algae |
(q) Mannitol |
(ii) 2-4, apical |
| (c) Green algae |
(r) Starch |
(iii) absent |
Options:
| 1. |
(a-r-ii) |
(b-p-iii) |
(c-q-i) |
| 2. |
(a-q-i) |
(b-p-iii) |
(c-r-ii) |
| 3. |
(a-q-i) |
(b-r-ii) |
(c-p-iii) |
| 4. |
(a-q-i) |
(b-p-ii) |
(c-r-iii) |
47. With reference to factors affecting the rate of photosynthesis, which of the following statements is not correct?
| 1. |
Light saturation for CO2-fixation occurs at 10% of full sunlight. |
| 2. |
Increasing atmospheric CO2 concentration by, up to, 0.05% can enhance the CO2-fixation rate |
| 3. |
C3 plants respond to higher temperatures with enhanced photosynthesis, while C4 plants have a much lower temperature optimum. |
| 4. |
Tomato is a greenhouse crop, which can be grown in a CO2-enriched atmosphere for a higher yield. |
48. The first step of decarboxylation in cellular respiration is:
1. Conversion of PGAL to DHAP
2. Conversion of PGAL to PGA
3. Conversion of Pyruvate to Acetyl CoA
4. Conversion of BPGA to PGA
49. Crossing over takes place between which chromatids and in which stage of the cell cycle?
| 1. |
Non-sister chromatids of non-homologous chromosomes at Zygotene stage of prophase I. |
| 2. |
Non-sister chromatids of homologous chromosomes at Pachytene stage of prophase I. |
| 3. |
Non-sister chromatids of homologous chromosomes at Zygotene stage of prophase I. |
| 4. |
Non-sister chromatids of non-homologous chromosomes at Pachytene stage of prophase I. |
50. Match the following:
| (a) |
Inhibitor of catalytic activity |
(i) |
Ricin |
| (b) |
Possess peptide bonds |
(ii) |
Malonate |
| (c) |
Cell wall material in fungi |
(iii) |
Chitin |
| (d) |
Secondary metabolite |
(iv) |
Collagen |
Choose the correct option from the following:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(iii) |
(i) |
(iv) |
(ii) |
| 2. |
(iii) |
(iv) |
(i) |
(ii) |
| 3. |
(ii) |
(iii) |
(i) |
(iv) |
| 4. |
(ii) |
(iv) |
(iii) |
(i) |
Zoology - Section A
51. Match the following columns and select the correct option:
|
Column-I |
|
Column-II |
| (a) |
Smooth endoplasmic
reticulum |
(i) |
Protein synthesis |
| (b) |
Rough endoplasmic
reticulum |
(ii) |
Lipid synthesis |
| (c) |
Golgi complex |
(iii) |
Glycosylation |
| (d) |
Centriole |
(iv) |
Spindle formation |
Options:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(ii) |
(i) |
(iii) |
(iv) |
| 2. |
(iii) |
(i) |
(ii) |
(iv) |
| 3. |
(iv) |
(ii) |
(i) |
(iii) |
| 4. |
(i) |
(ii) |
(iii) |
(iv) |
52. Consider the given two statements:
| Assertion (A): |
Gene therapy is a method of treating a disorder but cannot cure it. |
| Reason (R): |
Cells are drawn from a patient and the functional gene is introduced into these cells and transferred back to the patient. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
53. DNA fragments generated by the restriction endonucleases in a chemical reaction can be separated by:
1. Polymerase chain reaction
2. Electrophoresis
3. Restriction mapping
4. Centrifugation
54.
| Assertion (A): |
Bone marrow and thymus are primary lymphoid organs. |
| Reason (R): |
These are the organs, to which matured lymphocytes migrate, interact with antigens and then proliferate to become effector cells. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
55. Identify the correct statements:
| I: |
Cartilaginous fishes are ammonotelic. |
| II: |
Mammals are ureotelic. |
| III: |
Land snails are uricotelic. |
1. Only
I and
II
2. Only
I and
III
3. Only
II and
III
4.
I,
II and
III
56. GFR is regulated by:
| 1. |
JGA |
2. |
ADH |
| 3. |
OT |
4. |
HGP |
57. Which of the following helps in the regulation of the respiratory rhythm?
1. Respiratory rhythm centre
2. Pneumotaxic centre
3. Aortic arch and carotid artery receptors
4. All of the above
58. A canal called as the cerebral aqueduct passes through the:
| 1. |
Diencephalon |
2. |
Mid brain |
| 3. |
3rd ventricle |
4. |
4th ventricle |
59. Which of the following is an incorrect statement?
| 1. |
Hormones bind to specific proteins located in the target tissues only. |
| 2. |
Receptors for hormones can only be present on the cell membrane. |
| 3. |
Each receptor is specific to one hormone only. |
| 4. |
Nuclear receptors are intracellular receptors. |
60. Irregular thickening of arterial walls and narrowing of their lumen due to deposition of plaque is:
1. Atherosclerosis
2. Arteriosclerosis
3. Varicose vein
4. Rete Mirabile
61. Which of the following structure of protein is absolutely necessary for the many biological activities of proteins?
1. Primary
2. Secondary
3. Tertiary
4. Quaternary
62. What is true for open circulatory system?
| 1. |
Cells and tissues are directly bathed in blood |
| 2. |
Capillaries are present |
| 3. |
Blood is circulated only through a series of vessels of varying diameter |
| 4. |
Present in earthworm |
63. A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using:
| 1. |
Ligase |
2. |
Eco RI |
| 3. |
Taq polymerase |
4. |
polymerase II |
64. Mark out the correct statement regarding the characteristics of variants in a mixed population:
| 1. |
Industrialisation results in an increase in the types of variants. |
| 2. |
In a mixed population, those that can better adapt, survive and increase in population size. |
| 3. |
Less adapted variants will be completely wiped out. |
| 4. |
All of these |
65. Which of the following is an example of Mendelian disorder?
1. Klinefelter's syndrome
2. Down's syndrome
3. Turner's syndrome
4. Thalassemia
66. Extrusion of the second polar body from the egg nucleus occurs:
1. simultaneously with the first cleavage
2. after entry of sperm but before fertilization
3. after fertilization
4. before entry of sperm into the ovum
67.
| Assertion (A): |
A primer is a small segment of DNA that binds to a complementary strand of DNA. |
| Reason (R): |
Primers are necessary to stop the functioning of DNA polymerase enzyme and, therefore, are necessary in a polymerase chain reaction. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
68. Three of the following statements about enzymes are correct and one is wrong. Which one is wrong?
| 1. |
Most enzymes are proteins but some are lipids. |
| 2. |
Enzymes require optimum pH for maximal activity. |
| 3. |
Enzymes are denatured at high temperatures but in certain exceptional organisms they are effective even at temperatures 80°- 90°C. |
| 4. |
Enzymes are highly specific. |
69. Which one of the following pairs of animals are similar to each other pertaining to the feature stated against them?
| 1. |
Pteropus and Ornithorhyncus |
Viviparity |
| 2. |
Garden lizard and Crocodile |
Three chambered heart |
| 3. |
Ascaris and Ancylostoma |
Metameric segmentation |
| 4. |
Sea horse and Flying fish |
Cold-blooded (poikilothermal) |
70. Out of ‘X’ pairs of ribs in humans, only ‘Y’ pairs are true ribs. Select the option that correctly represents values of X and Y and provides their explanation:
|
X |
Y |
| 1. |
12 |
15 true ribs are attached dorsally to the vertebral column and sternum on the two ends |
| 2. |
24 |
7 true ribs are dorsally attached to the vertebral column but are free on the ventral side |
| 3. |
24 |
12 true ribs are dorsally attached to the vertebral column but are free on the ventral side |
| 4. |
12 |
7 true ribs are attached dorsally to the vertebral column and ventrally to the sternum |
71. Match each item in Column I with one in Column II and select your answer from the codes given:
|
Column I |
|
Column II |
| A. |
IUI |
a. |
injection of washed sperm into the uterus with a catheter. |
| B. |
ZIFT |
b. |
an egg fertilized in vitro (outside the body) is placed into a woman's fallopian tube. |
| C. |
GIFT |
c. |
eggs are removed from a woman's ovaries, and placed in one of the Fallopian tubes, along with the man's sperm. |
| D. |
ICSI |
d. |
injection of a single sperm directly into a mature egg. |
Codes:
|
A |
B |
C |
D |
| 1. |
a |
b |
c |
d |
| 2. |
d |
b |
c |
a |
| 3. |
a |
c |
b |
d |
| 4. |
d |
c |
b |
a |
72. Vasa recta in cortical nephrons:
| 1. |
Arises from afferent arteriole rather than efferent arteriole |
| 2. |
Does not get involved in counter-current exchange with the loop of Henle |
| 3. |
Carries deoxygenated blood rich in urea |
| 4. |
Is absent or highly reduced |
73. The group of neurosecretory cells found in hypothalamus is known as:
| 1. |
ganglia |
2. |
nuclei |
| 3. |
Tract |
4. |
nerve |
74. The correct match is:
75.
Select the correct option based on the statements below:
| Assertion (A): |
Spermatogenesis starts at the age of puberty. |
| Reason (R): |
Primary spermatocytes undergo second maturation division to become spermatids. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
76. Which of the following statements is not correct?
| 1. |
Lysosomes are formed by the process of packaging in the endoplasmic reticulum |
| 2. |
Lysosomes have numerous hydrolytic enzymes |
| 3. |
The hydrolytic enzyme of lysosomes are active under acidic pH |
| 4. |
Lysosomes are membrane bound structure |
77. What would be the heart rate of a person if the cardiac output is 5L, blood volume in the ventricles at the end of diastole is 100 mL and at the end, ventricular systole is 50 mL?
1. 125 beats per minute
2. 50 beats per minute
3. 75 beats per minute
4. 100 beats per minute
78. Identify the wrong statement with reference to the transport of oxygen.
| 1. |
Partial pressure of CO2 can interfere with O2 binding with haemoglobin. |
| 2. |
Higher H+ conc. in alveoli favours with the formation of oxyhaemoglobin. |
| 3. |
Low pCO2 in alveoli favours the formation of oxyhaemoglobin. |
| 4. |
Binding of oxygen with haemoglobin is mainly related to the partial pressure of O2. |
79. Which one of the following characteristic is incorrect with respect to cockroach?
| 1. |
In females, 7th - 9th sterna together form a genital pouch. |
| 2. |
10th abdominal segment in both sexes, bears a pair of anal cerci. |
| 3. |
A ring of gastric caeca is present at the junction of midgut and hind gut. |
| 4. |
Hypopharynx lies within the cavity enclosed by the mouth parts. |
80. A specific recognition sequence identified by endonucleases to make cuts at specific positions within the DNA is:
1. Palindromic Nucleotide sequences
2. Poly(A) tail sequences
3. Degenerate primer sequence
4. Okazaki sequences
81. Select the mismatch.
| 1. |
Rosie cow |
- |
1997 |
| 2. |
Patent on Basmati rice by an American company |
- |
1997 |
| 3. |
Human insulin production by Eli Lilly, an American company |
- |
1987 |
| 4. |
First clinical gene therapy |
- |
1990 |
82. Brain stem of the human brain consists of:
1. Mid-brain, Pons and Medulla Oblongata
2. Forebrain, Cerebellum and Pons
3. Thalamus, Hypothalamus and Corpora quadrigemina
4. Amygdala, Hippocampus and Corpus Callosum
83. Consider the given two statements:
| Assertion (A): |
Louis Pasteur, in his experiment, showed that in pre-sterilised flasks, life did not come from killed yeast while in another flask open to air, new living organisms arose from ‘killed yeast’. |
| Reason (R): |
Life comes only from pre-existing life. |
| 1. |
(A) is True but (R) is False |
| 2. |
Both (A) and (R) are True and (R) is the correct explanation of (A) |
| 3. |
Both (A) and (R) are True but (R) is not the correct explanation of (A) |
| 4. |
(A) is False but (R) is True |
84. Consider the given two statements:
| Assertion (A): |
Smoking is associated with increased incidence of cancers of lung, urinary bladder and throat. |
| Reason (R): |
Nicotine, present in tobacco, stimulates adrenal gland to release adrenaline and nor-adrenaline into blood circulation |
| 1. |
Both (A) and (R) are True and (R) correctly explains (A). |
| 2. |
(A) is True; (R) is False |
| 3. |
(A) is False; (R) is True |
| 4. |
Both (A) and (R) are True but (R) does not correctly explain (A). |
85. Which of the following events is likely to happen if diffusion of ions is allowed in a resting polarized axonal membrane?
1. Na+ will move out of the cell
2. Na+ will move into the cell
3. K+ will move into the cell
4. Na+ and K+ will both move out of the cell
Zoology - Section B
86. The volume of air expired forcefully after forceful inspiration is:
| 1. |
TV+ IRV |
2. |
TV+ERV+IRV |
| 3. |
TV+ERV+IRV+RV |
4. |
None of the above |
87. The metal present in hemoglobin is:
| 1. |
Magnesium |
2. |
Sodium |
| 3. |
Iron |
4. |
Copper |
88. The primary oocyte completes the first meiotic division at the stage of:
| 1. |
Primordial follicle |
2. |
Primary follicle |
| 3. |
Secondary follicle |
4. |
Tertiary follicle |
89. Given below are four statements A - D each with one or two blanks. Select the option which correctly fills up the blanks in two statements.
| A. |
Members of kingdom Animalia are (i) , all of them do not exhibit the same pattern of organisation of cells. Sponges exhibit (ii) level of organisation. |
| B. |
For sessile animals, (i) symmetry is advantageous, as it allows the food to be gathered from all sides. |
| C. |
Bilateral symmetry arose when animals on the ocean floor became mobile. A crawling animal is most likely to encounter food with the end that goes ahead. Head, enclosing the brain became associated with mouth end. This is called (i) . |
| D. |
Notochord is a (i) derived rod-like structure formed on the (ii) side during embryonic development in some animals. |
| 1. |
A |
B |
(i) Unicellular or multicellular
(ii) Cellular |
(i) Radial |
| 2. |
B |
C |
| (i) Bilateral |
(i) Cephalisation |
| 3. |
A |
D |
(i) Multicellular
(ii) Cellular |
(i) Mesodermally
(ii) Dorsal |
| 4. |
B |
D |
| (i) Bilateral |
(i) Ectodermally
(ii) Dorsal |
90. In human evolution, the development of the brain and language is an example of:-
1. Convergent evolution
2. Divergent evolution
3. Parallel evolution
4. Adaptive radiation
91. How many times can human urine be concentrated in a counter-current mechanism?
1. Four times
2. Five times
3. Three times
4. Two times
92. Find out the incorrect statement with respect to the metabolism of calcium in the human body:
| 1. |
High level of in the blood stimulates the thyroid gland's parafollicular cells to release calcitonin |
| 2. |
Calcitonin promotes deposition of into the bone matrix to reduce blood level |
| 3. |
Hypersecretion of PTH causes a deficiency of in the blood which increases the excitability of nerves and muscles and causes sustained muscular contractions (Tetany) |
| 4. |
Calcitriol hormone released from kidneys stimulates the absorption of in the gastrointestinal tract |
93. The anaerobic contraction of skeletal muscle causes the deposition or accumulation of:
1. Gluconic acid
2. Lactic acid
3. Pyruvic acid
4. Hydrochloric acid
94. The bioreactor shown in the diagram is:

1. Sparged stirred tank type
2. Airlift type
3. Simple stirred tank type
4. Fluidized bed type
95. Maximum number of existing transgenic animals is of:
1. Fish
2. Mice
3. Cow
4. Pig
96. The phase of the menstrual cycle in humans that last for 7-8 days is:
1. follicular phase
2. ovulatory phase
3. luteal phase
4. menstruation
97. Natural methods of birth control include:
1. Abstinence
2. Coitus interruptus
3. Lactational amenorrhoea
4. All of these
98. Gambusia is a …….. that feeds on ..….
| 1. |
Mosquito, larvae |
| 2. |
Fish, mosquito-larvae |
| 3. |
Frog species, mosquito of stagnant water |
| 4. |
Snail, Protozoan |
99. Which organism has more base pairs?
1. E.coli
2. Bacteriophage
3. human
4. Yeast
100. Consider the two given statements:
| Assertion (A): |
Foreign and vector DNA must be cleaved with the same restriction enzyme when making recombinant DNA. |
| Reason (R): |
Each restriction enzyme cuts DNA at a specific cleavage site. |
| 1. |
Both (A) and (R) are True and (R) correctly explains (A). |
| 2. |
(A) is True; (R) is False |
| 3. |
(A) is False; (R) is True |
| 4. |
Both (A) and (R) are True but (R) does not correctly explain (A). |
Chemistry - Section A
101. Which reagent is used to convert benzoic acid into benzoyl chloride?
1. Cl2, hv
2. SO2
3. SOCl2
4. Cl2, H2O
102. Considering that , the magnetic moment (in BM) of would be:
1. 0
2. 4.9
3. 6.9
4. 3.5
103. Consider the following chemical reaction,
\(\mathrm{CH} \equiv \mathrm{CH}\xrightarrow[(2)~~ \mathrm{CO}, ~\mathrm{HCl}, ~\mathrm{AlCl}_3]{\text { (1) } \text { Red hot / Fe tube, } 873 \mathrm{~K}}~~~\text{Product}\)
The number of sp2 hybridized carbon atom(s) present in the product is:
1. 6
2. 7
3. 8
4. 5
104. The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are:
1. Cl– and ClO–
2. Cl– and ClO2–
3. ClO– and ClO3–
4. ClO3– and ClO3–
105. For the reaction:
\(FeO_{(s)} + CO_{(g)} \rightleftharpoons Fe_{(s)} + {CO_2}_{(g)}, K_p = 0.265 \) at 1050 K. If the initial partial pressures are: \(P_{CO}\)= 1.4 atm and \(P_{CO_2}\)= 0.80 atm, the partial pressure of CO2 at equilibrium at 1050 K would be:
| 1. |
4.61 atm |
2. |
1.74 atm |
| 3. |
0.46 atm |
4. |
0.17 atm |
106. The term anomers of glucose refer to:
| 1. |
Isomers of glucose that differ in configurations at carbons one and four (C-1 and C-4) |
| 2. |
A mixture of (D)-glucose and (L)-glucose |
| 3. |
Enantiomers of glucose |
| 4. |
Isomers of glucose that differ in configuration at carbon one (C-1) |
107. In a mixture of A and B, components show negative deviations when:
| 1. |
A-B interaction is stronger than A-A and B-B interaction |
| 2. |
A-B interaction is weaker than A-A and B-B interaction |
| 3. |
\(\Delta V_{\text {mix }}>0, \quad \Delta S_{\text {mix }}>0\) |
| 4. |
\(\Delta V_{\text {mix }}=0, \quad \Delta S_{\text {mix }}>0\) |
108. The number of atoms in 560 g of Fe (atomic mass 56 g mol–1) is:
| 1. |
Twice that of 70 g N |
| 2. |
Half that of 20 g H |
| 3. |
Both 1 and 2 |
| 4. |
None of the above |
109. According to the periodic law of elements, the variation in properties of elements is related to their:
1. Atomic masses
2. Nuclear mass
3. Atomic numbers
4. Nuclear neutron-proton number ratio
110. Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labeled as A and B respectively for testing. A and B were separately taken in a test tube and boiled with NaOH solution. The end solution in each test tube was made acidic with dilute HNO3 and then some AgNO3 was added. A substance present in test tube B gave a yellow precipitate. The true statement among the following regarding the experiment is:
1. A was C6H5I
2. A was C6H5CH2I
3. B was C6H5I
4. Addition of HNO3 was unnecessary
111. The elementary step of the reaction, \(2 \mathrm{Na}+\mathrm{Cl}_2\rightarrow2 \mathrm{NaCl}\) is found to follow 3rd order kinetics. Its molecularity is:
| 1. |
One(1) |
2. |
Two(2) |
| 3. |
Three(3) |
4. |
Four(4) |
112. Identify the compound B in the reaction sequence shown below:

114. Consider the following reaction,
XeF6 + 2H2O → X + 4HF
The product X is:
1. XeO2F2
2. XeO3
3. XeOF4
4. None of the above
115. Select the correct option based on statements below:
| I: |
[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic. |
| II: |
[Fe(H2O)6]3+ has 4 unpaired electrons while [Fe(CN)6]3− has 5 unpaired electrons. |
1. Both I and II are true.
2. I is true and II is false.
3. Both I and II are false.
4. I is false but II is true.
116. The noble gas that does not occur in the atmosphere is :
1. He
2. Kr
3. Ne
4. Rn
117. The ionization constant of some weak bases at a particular temperature is given below:
| Base |
Dimethylamine |
Urea |
Pyridine |
Ammonia |
| Kb |
5.4 × 10-4 |
1.3 × 10-14 |
1.77 × 10-9 |
1.77 × 10-5 |
The decreasing order of the bases on the extent of their ionization at equilibrium is:
| 1. |
Urea > Ammonia > Dimethylamine > Pyridine |
| 2. |
Ammonia > Dimethylamine > Pyridine > Urea |
| 3. |
Pyridine > Urea > Dimethylamine > Ammonia |
| 4. |
Dimethylamine > Ammonia > Pyridine > Urea |
118. Match the compounds in column I with the oxidation states of Cl in column II.
| Column I(Compound) |
Column II(Oxidation state) |
| a. Cl2O7 |
i. +4 |
| b. NaClO3 |
ii. +1 |
| c. Cl2O |
iii. +5 |
| d. ClO2 |
iv. +7 |
Options:
|
a |
b |
c |
d |
| 1. |
iii |
iv |
ii |
i |
| 2. |
ii |
iii |
iv |
i |
| 3. |
iv |
iii |
ii |
i |
| 4. |
iv |
i |
iii |
ii |
119. In the following sequence of reactions :
\(CH_3CH_2OH \xrightarrow{KMnO_4}(a) \xrightarrow{SOCl_2, ~NH_3}(b)\xrightarrow[]{Br_{2}, \ NaOH}(C)\)
The end product (c) is:
1. Acetone
2. Ethylamine
3. Acetic acid
4. Methylamine
120. Major product of the reaction is:

1. 2,4- Dinitrophenol
2. 3,4-Dinitrophenol
3. 2,4,6-Trinitrophenol
4. 3,4,5-Trinitrophenol
121. The ground state magnetic property of B2 and C2 molecules will be :
1. B2 paramagnetic and C2 diamagnetic
2. B2 diamagnetic and C2 paramagnetic
3. Both are diamagnetic
4. Both are paramagnetic
122. Among the following, the conformation that corresponds to the most stable conformation of meso-butane-2,3-diol is:
123. Which of the following is not involved in the titration of Mohr's salt (
\(\mathrm{FeSO}_4\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}\)) with potassium permanganate (
\(\mathrm{KMnO}_4\))?
| 1. |
Oxidation |
2. |
Reduction |
| 3. |
Precipitation |
4. |
Acid-base reaction |
124. Which of the following statement is false for fuel cells?
1. They are more efficient.
2. They are free from pollution.
3. They run till reactants are active.
4. They are examples of electrolytic cells.
125. The possible correct set of quantum numbers for the unpaired electron of
\(Cl\) atom is:
| 1. |
\(2,\ 0,\ 0,\ +\frac12 \) |
2. |
\(2,\ 1,\ -1,\ +\frac12\) |
| 3. |
\(3,\ 1,\ +1,\ +\frac12 \) |
4. |
\(3,\ 0,\ +2,\ \frac12\) |
126. Which of the following equation represents a reaction that provides the enthalpy of the formation of CH
3Cl?
| 1. |
\(\mathrm{C}(s)+\mathrm{HCl}(g)+\mathrm{H}_{2}(g) \longrightarrow \mathrm{CH}_{3} \mathrm{Cl}(g)\) |
| 2. |
\(\mathrm{C}(\mathrm{s})+3 \mathrm{H}(\mathrm{g})+\mathrm{Cl}(g) \longrightarrow \mathrm{CH}_{3} \mathrm{Cl}(g)\) |
| 3. |
\(\mathrm{C}(\mathrm{s})+3 / 2 \mathrm{H}_{2}(g)+1 / 2 \mathrm{Cl}_{2}(g) \longrightarrow \mathrm{CH}_{3} \mathrm{Cl}(g) \) |
| 4. |
\(\mathrm{CH}_{4}(g)+\mathrm{Cl}_{2}(g) \longrightarrow \mathrm{CH}_{3} \mathrm{Cl}(g)+\mathrm{HCl}(g)\) |
127. The first ionization enthalpies of elements X and Y are 419 kJ mol
–1 and 590 kJ mol
–1, respectively and the second ionization enthalpies of X and Y are 3069 kJ mol
–1 and 1145 kJ mol
–1, respectively. The correct statement is:
| 1. |
X is an alkali metal and Y is an alkaline earth metal. |
| 2. |
X is an alkaline earth metal and Y is an alkali metal. |
| 3. |
Both X and Y are alkali metals. |
| 4. |
Both X and Y are alkaline earth metals. |
128. The half-life of a first-order reaction is 2000 years. If the concentration after 8000 years is 0.02 M, then the initial concentration was:
1. 0.16 M
2. 0.32 M
3. 0.08 M
4. 0.04 M
129. The mass of a 2.5 mL solution (the density of the solution is 2.15 g/mL) in the correct significant figures is:
| 1. |
5375 × 10–3 g |
2. |
5.4 g |
| 3. |
5.38 g |
4. |
53.75 g |
130. Which of the following methods is incorrect for the synthesis of alkenes?
1. Treatment of alkynes with Na in liquid NH3
2. Heating alkyl halides with alcoholic KOH
3. Treating alkyl halides in aqueous KOH solution
4. Treating vicinal dihalides with Zn metal
131. The standard Gibbs free energy for the following reaction is:
\(Fe^{2+} (aq)+Ag^+(aq) \rightarrow Fe^{3+}(aq)+Ag(s)\)
Given:
\(\small(E^o_{Ag^+/Ag} = 0.80 V; E^o_{Fe^{3+}/Fe^{2+}} = 0.77 V; E^o_{Fe^{2+}/Fe} = 0.44 V )\ \).
| 1. |
- 2.895 kJ/mol |
2. |
- 2.895 J/mol |
| 3. |
- 239.287 kJ/mol |
4. |
239.287 kJ/mol |
132. The correct IUPAC name for [CrF
2(en)
2]Cl is:
| 1. |
Chlorodifluoridoethylenediaminechromium(III) chloride |
| 2. |
Bis-(ethylenediamine)difluoridochromium(III) chloride |
| 3. |
Difluorobis-(ethylenediamine)chromium(III) chloride |
| 4. |
Chlorodifluoridobis(ethylenediamine)chromium (III) |
133. Which of the following sets represents a complex and a double salt, respectively?
1. CuSO4.5H2O and CuCl2.4NH3
2. PtCl2.2NH3 and PtCl4.2HCl
3. K2PtCl2.2NH3 and KAl(SO4)2.12H2O
4. NiCl2.6H2O and NiCl2(H2O)4
134. A reaction among the following can generate isonitriles as a major product.
A.
\(R-X + HCN \rightarrow \)
B.
\(R-X + AgCN \rightarrow \)
C.
\(R-X + KCN \rightarrow \)
D.
\(R-X + NaCN \xrightarrow [C_2H_5OH] {H_2O} \)
Choose the most appropriate answer from the options given below:
| 1. |
(D) only |
2. |
(C) and (D) only |
| 3. |
(B) only |
4. |
(A) and (B) only |
135. Outer orbital complex salt(s) among the following is/are:
| A. \(\mathrm{\left[{Mn}({CN})_6 \right]^{3-}}\) |
B. \(\mathrm{\left[{C}{{o}}({C}_2 {O}_4)_3\right]^{3-}}\) |
| C. \(\mathrm{\left[{MnCl}_6\right]^{3-}}\) |
D. \(\mathrm{\left[{Ni}({H}_2 {O})_6\right]^{2+}} \) |
Choose the correct answer from the options given below:
| 1. |
A and B only |
2. |
B and C only |
| 3. |
C and D only |
4. |
C only |
Chemistry - Section B
136. Number of isomers of complex are:
137. General behavior of is:
1. To give electrons
2. To Give
3. Reaction with
4. To accept electrons
138. The major products of the following reaction are:

139. The molecule in which hybrid MOs involve only one d-orbital of the central atom is:
| 1. |
\(X e F_{4}\) |
2. |
\(\left[ N i (CN)_{4} \right]^{2 -}\) |
| 3. |
\(B r F_{5}\) |
4. |
\(\left[ C r F_{6} \right]^{3 -}\) |
140. Phenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:
141. C6H6 freezes at 5.5°C. The temperature at which a solution of 10 g of C4H10 in 200 g of C6H6 will freeze is:
(The molal freezing point depression constant of C6H6 is 5.12°C/m.)
| 1. |
2 °C |
2. |
1 °C |
| 3. |
6 °C |
4. |
5 °C |
142. The correct expression for the following reaction is:
Fe2N(s) + \(\frac{3}{2}\)H2(g) \(\leftrightharpoons \) 2Fe(s) + NH3(g)
| 1. |
|
2. |
|
| 3. |
|
4. |
|
143. A solution of phenol in chloroform when treated with aqueous NaOH gives compound P as a major product. The mass percentage of carbon in P is:
(to the nearest integer) (Atomic mass : C =12; H=1; O=16)
144. The rate of a reaction is decreased by 3.555 times when the temperature was changed from 40°C to 30°C. The activation energy (in kJ ) of the reaction is:
(Take R=8.314 J In 3.555=1.268)
1. 100 kJ/mol
2. 120 kJ/mol
3. 95 kJ/mol
4. 108 kJ/mol
145. Given
\(\begin{aligned} &\mathrm{{C}_{{(graphite) }}+{O}_{2}({~g})} → \mathrm{{CO}_{2}({~g})} \\ &\mathrm{\Delta_{r} {H}^{\circ}=-393.5 {~kJ} {~mol}^{-1}} \\ &\mathrm{H_{2}(g) + \frac{1}{2} {O}_{2}({~g})} → \mathrm{{H}_{2} {O}({l})} \\ &\mathrm{\Delta_{r} {H}^{\circ}=-285.8 {~kJ} {~mol}^{-1}} \\ &\mathrm{{CO}_{2}({~g})+2 {H}_{2} {O}({l})} → \mathrm{{CH}_{4}({~g})+2 {O}_{2}({~g})} \\ &\mathrm{\Delta_{r} {H}^{\circ}=+890.3 {~kJ} {~mol}^{-1}} \end{aligned}\)
Based on the above thermochemical equations, the value of ΔrH° at 298 K for the reaction
\(\mathrm{C_{(graphite)} + 2 H_{2} (g) → CH_{4} (g)}\) will be :
1. –74.8 kJ mol–1
2. –144.0 kJ mol–1
3. +74.8 kJ mol–1
4. +144.0 kJ mol–1
146. The reduction potential of hydrogen half-cell will be negative if:
1. P(H2) = 1atm and [H+] = 2.0 M
2. P(H2) = 1 atm and [H+] = 1.0 M
3. P(H2) = 2 atm and [H+] = 1.0 M
4. P(H2) = 2 atm and [H+] = 2.0 M
147. Which of the following pairs are metamers of ethyl propionate?
1. C4H9COOH and HCOOC4H9
2. C4H9COOH and CH3COOC3H7
3. CH3COOCH3 and CH3COOC3H7
4. CH3COOC3H7 and C3H7COOCH3
148. The molecular shapes of SF4, CF4, and XeF4 are:
| 1. |
Different with 1, 0, and 2 lone pairs of electrons on the central atom, respectively |
| 2. |
Different with 0, 1, and 2 lone pairs of electrons on the central atom, respectively |
| 3. |
The same with 1, 1, and 1 lone pair of electrons on the central atoms, respectively |
| 4. |
The same with 2, 0, and 1 lone pair of electrons on the central atom, respectively |
149. A hydrocarbon of molecular formula C6H10 reacts with sodamide and the same on ozonolysis followed by hydrogen peroxide oxidation gives two molecules of carboxylic acids, one being optically active. The hydrocarbon maybe:
| 1. |
1-Hexyne |
2. |
3-Hexyne |
| 3. |
3-Methyl-1-pentyne |
4. |
3,3-Dimethyl-1-butyne |
150. The ammonia evolved from the treatment of 0.30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0.1 M sulphuric acid. The excess acid required 20 mL of 0.5 M sodium hydroxide solution for complete neutralization. The percentage of nitrogen in the organic compound is:
1. 46.6 %
2. 50.4 %
3. 42.8 %
4. 40.5 %
Physics - Section A
151. A stationary horizontal disc is free to rotate about its axis. When a torque is applied on it, its kinetic energy as a function of \(\theta,\) where \(\theta\) is the angle by which it has rotated, is given as \(k\theta^2\) (where \(k\) is constant). If its moment of inertia is \(I,\) then the angular acceleration of the disc is:
1. \(\frac{k}{I} \theta\)
2. \(\frac{k}{2 I} \theta\)
3. \(\frac{k}{4 I} \theta\)
4. \(\frac{2 k}{I} \theta\)
152. A particle is moving along a circular path with a uniform speed. Through what angle does its angular velocity change when it completes half of the circular path?
| 1. |
\(0^\circ\) |
2. |
\(45^\circ\) |
| 3. |
\(180^\circ\) |
4. |
\(360^\circ\) |
153. A conductor rod
\(AB\) moves parallel to
\(x\)-axis in a uniform magnetic field, pointing in the positive
\(z\)-direction. The end
\(A\) of the rod gets:
| 1. |
positively charged |
| 2. |
negatively charged |
| 3. |
neutral |
| 4. |
first positively charged and then negatively charged |
154. An alternating current is given by the equation;
\({I}{=}{I}_{1}\cos\mathit{\omega}{t}{+}{I}_{2}\sin\mathit{\omega}{t}.\) The RMS current is given by:
| 1. |
\(\dfrac{I_1+I_2}{\sqrt2}\) |
2. |
\(\dfrac{(I_1+I_2)^2}{\sqrt2}\) |
| 3. |
\(\dfrac{{\left({{I}_{1}^{2}{+}{I}_{2}^{2}}\right)}^{1/2}}{\sqrt{2}}\) |
4. |
\(\dfrac{1}{2}{\left({{I}_{1}^{2}{+}{I}_{2}^{2}}\right)}^{1/2}\) |
155. A convex lens has \(20~\mathrm{cm}\) focal length in air. What will be its focal length when placed in a transparent liquid?
(take \(\mu_\text{liquid}=1.3,\mu_\text{glass}=1.5\))
1. \(20~\text{cm}\)
2. \(65~\text{cm}\)
3. \(30~\text{cm}\)
4. \(104~\text{cm}\)
156. The refractive index of glass is \(1.9\). If light travels through a glass slab of thickness \(d\) in time \(t\) and takes the same time to travel through a transparent beaker filled with water upto a thickness \(1.5~d\), then the refractive index of water is:
1. \(1.27\)
2. \(1.33\)
3. \(1.20\)
4. \(1.50\)
157. The energy stored in the choke coil is in the form of:
1. heat
2. electric energy
3. magnetic energy
4. chemical energy
158. In a purely resistive AC circuit, which of the following sketches represents the variation of the current amplitude
\(I_0\) with the frequency
\({\omega}?\)
159. A uniform rod of weight
\(W_1\) is suspended from the ceiling, and an additional weight
\(W_2\) hangs from its lower end. If the rod has a cross-sectional area
\(A,\) what is the stress at its midpoint?
| 1. |
\(\dfrac{\left({{W}_{1}{+}{W}_{2}}\right)}{A}\) |
2. |
\(\dfrac{\left({{W}_{1}{-}{W}_{2}}\right)}{A}\) |
| 3. |
\(\dfrac{\left({{W}_{1}/2}\right){+}{W}_{2}}{A}\) |
4. |
\(\dfrac{\left({{W}_{2}/2}\right){+}{W}_{1}}{A}\) |
160. An infinite non-conducting sheet has a surface charge density \(\sigma=0.1 ~\mu\text{C m}^{-2} \) on one side. How far apart are equipotential surfaces whose potentials differ by \(50~\text V?\)
1. \(8.8~\text{mm}\)
2. \(8.8~\text{cm}\)
3. \(8.8~\mu\text{m}\)
4. \(8.8~\text{pm}\)
161. Two unit negative charges are placed on a straight line. A positive charge \(q\) is placed exactly at the mid-point between these unit charges. If the system of three charges is in equilibrium, the value of \(q\) (in C) is:
1. \(1.0\)
2. \(0.75\)
3. \(0.5\)
4.\(0.25\)
162. A small bar magnet has points
\(A\) and
\(B\) along its axis at distances of
\(24~\text{cm}\) and
\(48~\text{cm}\) on the opposite sides. The ratio of magnetic fields at these points will be:

| 1. |
\(8\) |
2. |
\(\frac{1}{2\sqrt{2}}\) |
| 3. |
\(3\) |
4. |
\(4\) |
163. Displacement current exists:
| 1. |
when an electric field is changing in the circuit. |
| 2. |
when an electric field is constant. |
| 3. |
when an electric field is absent. |
| 4. |
always exists independent of the electric field. |
164. The ratio of the areas within the electron orbits for the first excited state to the ground state for the hydrogen atom is:
| 1. |
\(2:1\) |
2. |
\(4:1\) |
| 3. |
\(8:1\) |
4. |
\(16:1\) |
165. When an unpolarised light beam is incident from the air onto glass
\((n= 1.5)\) at the polarising angle, then:
| 1. |
reflected beam is polarised \(100\) percent. |
| 2. |
reflected and refracted beams are partially polarised. |
| 3. |
the reason for (1) is that almost all the light is reflected. |
| 4. |
all of the above. |
166. The radius of a ball is
\(\left({{5}{.}{2}\pm{0}{.}{2}}\right)\) cm. The percentage error in the volume of the ball is:
| 1. |
\(11 \text{%}\) |
2. |
\(4 \text{%}\) |
| 3. |
\(7 \text{%}\) |
4. |
\(9 \text{%}\) |
167. A body sliding on a smooth inclined plane requires \(4~\text{s}\) to reach the bottom starting from rest at the top. How much time does it take to cover one-fourth the distance starting from rest at the top?
1. \(1~\text{s}\)
2. \(2~\text{s}\)
3. \(4~\text{s}\)
4. \(16~\text{s}\)
168. A conductor of resistance
\(3~\Omega\) is stretched uniformly till its length is doubled. The wire is now bent in the form of an equilateral triangle. The effective resistance between the ends of any side of the triangle (in ohm) is:
| 1. |
\(\dfrac{9}{2}\) |
2. |
\(\dfrac{8}{3}\) |
| 3. |
\(2\) |
4. |
\(1\) |
169. How much work per kilogram needs to be done to shift a
\(1\) kg mass from the surface of the earth to infinity? (Take acceleration due to gravity
\(=g\) and radius of the earth
\(=R\).)
| 1. |
\(\dfrac{g}{R}\) |
2. |
\(\dfrac{R}{g}\) |
| 3. |
\(gR\) |
4. |
\(\dfrac{g}{R^{2}}\) |
170. A thermos flask is polished well:
| 1. |
to make it attractive |
| 2. |
for shining |
| 3. |
to absorb all radiation from outside |
| 4. |
to reflect all radiation from outside |
171. A long straight wire carries \(10~\text{A}\) dc current. An electron travels perpendicular to this wire at a distance of \(0.1~\text{m}\) with velocity \(5.0 \times 10^{6}~\text{ms}^{-1}\). The magnitude of the force acting on the electron due to the current in the wire is:
1. zero
2. \(0.6 \times 10^{-17}~\text{N}\)
3. \(1.6 \times 10^{-17}~\text{N}\)
4. \(2.2 \times 10^{-17}~\text{N}\)
172. The current range of an ammeter of \(1~\text{A}\) is obtained by shunting a \(27~\Omega\) galvanometer with a \(3~\Omega\) resistance. What is the current rating of the galvanometer?
1. \(0.1~\text{A}\)
2. \(0.2~\text{A}\)
3. \(0.3~\text{A}\)
4. \(0.4~\text{A}\)
173. An electron accelerated under a potential difference
\(V\) volt has a certain wavelength
\(\lambda\). Mass of proton is some
\(2000\) times of the mass of the electron. If the proton has to have the same wavelength
\(\lambda\), then it will have to be accelerated under a potential difference of:
| 1. |
\(V\) volts |
2. |
\(2000V\) volts |
| 3. |
\(\dfrac{V}{2000} \) volts |
4. |
\(\sqrt{2000}V\) volts |
174. In a semiconductor the forbidden energy gap between the valence band and the conduction band is of the order of:
| 1. |
\(1~\text{eV}\) |
2. |
\(5~\text{eV}\) |
| 3. |
\(1~\text{keV}\) |
4. |
\(1~\text{MeV}\) |
175. A person takes an aim at a monkey sitting on a tree and fires a bullet. If on seeing the smoke the monkey begins to fall freely, then the bullet will:
| 1. |
hit the monkey. |
| 2. |
go above the monkey. |
| 3. |
go below the monkey. |
| 4. |
hit the monkey if the initial velocity of the bullet is more than a definite velocity. |
176. A spring gun of spring constant
\(90~\text{N/cm}\) is compressed
\(12~\text{cm}\) by a ball of mass
\(16~\text{g}\). If the trigger is pulled, the velocity of the ball is:
| 1. |
\(50~\text{ms}^{-1}\) |
2. |
\(9~\text{ms}^{-1}\) |
| 3. |
\(40~\text{ms}^{-1}\) |
4. |
\(90~\text{ms}^{-1}\) |
177. A shell explodes and many pieces fly off in different directions. Which of the following is conserved?
| 1. |
Kinetic energy |
| 2. |
Momentum |
| 3. |
Neither momentum nor kinetic energy |
| 4. |
Momentum and kinetic energy |
178. Suppose the Earth suddenly contracts such that the duration of the new day is \(24/n^2\). If the original radius is \(r\), then the new radius assuming that the mass of Earth remains the same will be:
1. \(r/n^2\)
2. \(nr\)
3. \(r/n\)
4. \(2nr\)
179. In the
\(\mathrm{P\text-V}\) diagram,
\(\mathrm{I}\) is the initial state and
\(\mathrm{F}\) is the final state. The gas goes from
\(\mathrm{I}\) to
\(\mathrm{F}\) by;
(i) \(\mathrm{IAF}\) (ii) \(\mathrm{IBF}\) (iii) \(\mathrm{ICF}\)
The heat absorbed by the gas is:

| 1. |
the same in all three processes. |
| 2. |
the same in (i) and (ii). |
| 3. |
greater in (i) and (ii) than in (iii). |
| 4. |
the same in (i) and (iii). |
180. An ideal gas (\(\gamma=1.51\)) is expanded adiabatically. How many times has the gas to be expanded to reduce the root mean square velocity of molecules \(2.0\) times?
1. \(4\) times
2. \(16\) times
3. \(8\) times
4. \(2\) times
181. Given \(x=x_m\text{cos}(\omega t+\phi),\) if at \(t=0,\) \(x = x_m,\) then the time taken to reach \(x = \dfrac{x_m}{2}\) is:
(where \(T\) is the time period of the motion)
1. \(3T/2\)
2. \(T/3\)
3. \(2T/3\)
4. \(T/6\)
182. By sucking through a straw, a student can reduce the pressure in his lungs to
\(750~\text{mm}\) of
\(\mathrm{Hg}\) (density = \(13.6~\text{g/cm}^3\)). Using the straw, he can drink water from a glass up to a maximum depth of:
| 1. |
\(10~\text{cm}\) |
2. |
\(75~\text{cm}\) |
| 3. |
\(13.6~\text{cm}\) |
4. |
\(1.36~\text{cm}\) |
183. The current flowing through the diode
\(D_2\) is:

1. zero
2.
\(4~\text{mA}\)
3.
\(2.5~\text{mA}\)
4.
\(0.8~\text{A}\)
184. Given below are two statements:
| Statement I: |
The speed of sound waves in a medium depends on the elastic modulus and the density of the medium. |
| Statement II: |
The speed of sound in a gas is independent of its temperature. |
| 1. |
Statement I is incorrect and Statement II is correct. |
| 2. |
Both Statement I and Statement II are correct. |
| 3. |
Both Statement I and Statement II are incorrect. |
| 4. |
Statement I is correct and Statement II is incorrect. |
185. Which of the following particles, is unstable?
1. Proton
2. Neutron
3. Electron
4. Antineutrino
Physics - Section B
186. The initial velocity \(v_i\) required to project a body vertically upwards from the surface of the earth to just reach a height of \(10R\), where \(R\) is the radius of the earth, described in terms of escape velocity \(v_e\) is:
| 1. |
\(\sqrt{\dfrac{10}{11}}v_e\) |
2. |
\(\sqrt{\dfrac{11}{10}}v_e\) |
| 3. |
\(\sqrt{\dfrac{20}{11}}v_e\) |
4. |
\(\sqrt{\dfrac{11}{20}}v_e\) |
187. A particle is thrown with a velocity of \(\mathrm{u}\) m/s. If it passes through A and B as shown in the figure at time \(t_1=1~\text{s}\) and \(t_2=3~\text{s}\), then the value of \(\mathrm{h}\) is: ( Take \(g=10~\text{m/s}^{2}\))

| 1. |
\(15~\text{m}\) |
2. |
\(10~\text{m}\) |
| 3. |
\(30~\text{m}\) |
4. |
\(20~\text{m}\) |
188. The deflection of a moving coil galvanometer falls from
\(60\) divisions to
\(12\) divisions for the same value of current in the circuit, when a shunt of
\(12~\Omega\) is connected. The resistance of the galvanometer is:
| 1. |
\(2~\Omega\) |
2. |
\(20~\Omega\) |
| 3. |
\(48~\Omega\) |
4. |
\(96~\Omega\) |
189. A particle of mass \(m\) is moving in a circular path of constant radius \(r\) such that its centripetal acceleration \(a_c\) is proportional to \(t^n\) (where \(t\) is time). The power \(P\) is proportional to:
1. \(t^n\)
2. \(t^{(n-2)}\)
3. \(t^{(n-1)}\)
4. \({t}^{\left({{n}{/}{2}{-}{1}}\right)}\)
190. In an insulator, the energy gap between the conduction band and the valence band is:
1. infinity
2. wide
3. narrow
4. zero
191. Concave and convex lenses are placed touching each other. The ratio of magnitudes of their power is \(2:3\). If the focal length of the system is \(30~\text{cm}\), then the focal lengths of individual lenses are (in cm):
1. \(-75,50\)
2. \(-15,10\)
3. \(75,50\)
4. \(75,-50\)
192. A small spherical ball cannot hold one coulomb of charge in air because at this stage:
| 1. |
voltage of the ball becomes high enough to destroy the insulating property of air. |
| 2. |
capacity of the ball is too high to hold the charge. |
| 3. |
nuclear forces wipe off the charge of the ball. |
| 4. |
electromagnetic forces reduce the charge of the ball. |
193. Liquid drops are falling slowly one by one from a vertical glass tube. Establish a relation between the weight
\(W\) of a drop, the surface tension
\(T,\) and the radius
\(r\) of the tube:
\(\left({\mathit{\theta}{=}\mathop {0}\nolimits^{\circ}}\right)\)
| 1. |
\(W=\pi r^{2}T\) |
2. |
\(W=2\pi rT\) |
| 3. |
\(W=2\pi r^{2}T \) |
4. |
\(W=\frac{4}{3}(\pi r^{3}T)\) |
194. The heat energy absorbed by a system in going through a cyclic process shown in the figure is:

| 1. |
\({10}^{3}\mathit{\pi}\) J |
2. |
\({10}^{2}\mathit{\pi}\) J |
| 3. |
\({10}^{4}\mathit{\pi}\) J |
4. |
\({10}^{7}\mathit{\pi}\) J |
195. The total energy of a particle executing simple harmonic motion is:
1. \(\propto{x}\)
2. \(\propto{x}^{2}\)
3. independent of \(x \)
4. \(\propto{x}^{1/2}\)
(where \(x\) is the displacement from the mean position)
196. A wave represented by the equation \(y=a\cos\left(kx+\omega t\right)\) is superimposed on another wave to form a stationary wave such that the point \(x=0\) is a node. The equation for the other wave is:
1. \(a\sin\left(kx+\omega t\right)\)
2. \(-a\cos\left(kx+\omega t\right)\)
3. \(-a\cos\left(kx-\omega t\right)\)
4. \(-a\sin\left(kx-\omega t\right)\)
197. \(X\) is a battery of emf
\(6~\text{V}\) and internal resistance
\(1\text{ ohm}.\) The potential at point
\(P\) in the figure is:
1.
\(6~\text{V}\)
2.
\(5~\text{V}\)
3.
\(3~\text{V}\)
4.
\(2~\text{V}\)
198. The threshold frequency for a certain metal is \(\nu_{0}.\) When the light of frequency \(\nu~=2\nu_{0}\) is incident on it, the maximum velocity of photoelectrons is \({4}\times{10}^{6}~\text{m/s}.\) If the frequency of incident radiation is increased to \(5\nu_{0},\) then the maximum velocity of photoelectrons (in \(\text{m/s}\)) will be:
1. \(\left({4/5}\right)\times{10}^{6}\)
2. \({2}\times{10}^{6}\)
3. \({8}\times{10}^{6}\)
4. \({2}\times{10}^{7}\)
199. A boy of mass \(40\) kg is hanging from the horizontal branch of a tree. The tension in his arms is minimum when the angle between the arms is:
1. \(0^\circ\)
2. \(90^\circ\)
3. \(120^\circ\)
4. \(180^\circ\)
200. The ratio of the moments of inertia of two spheres, about their diameters, having the same mass and their radii being in the ratio of \(1:2\), is:
| 1. |
\(2:1\) |
2. |
\(4:1\) |
| 3. |
\(1:2\) |
4. |
\(1:4\) |
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course




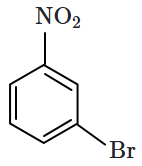
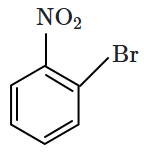
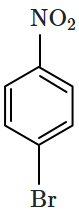
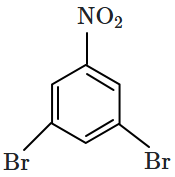




 1. 2,4- Dinitrophenol
1. 2,4- Dinitrophenol 


















 1. zero
1. zero
