Which of the following is an activator for both ribulose biphosphate carboxylase-oxygenase and phosphoenol pyruvate carboxylase?
1. Zinc
2. Magnesium
3. Manganese
4. Molybdenum
Which of the following statements regarding Euglenoids is not true?
| 1. | Instead of a cell wall, they have a lipid rich pellicle |
| 2. | They have two flagella, a short and a long one |
| 3. | They are photosynthetic in the presence of sunlight but behave like a heterotroph when deprived of sunlight |
| 4. | Pigments of Euglenoids are identical to those present in higher plants |
Consider the following statements:
I. Deoxyribonucleoside triphosphates act as substrates as well as provide energy for polymerization reaction during DNA replication.
II. The replication of DNA is both semiconsenvative and semi- discontinuous
III. In bacterial DNA replication there are multiple ori and replication fork moves bi-directionally
Which of the above statements are true?
1. I and II only
2. I and III only
3. II and III only
4. I, II and III
In bryophytes and pteriodophytes, transport of male gametes requires
1. insects
2. birds
3. water
4. wind
Male and female gametophytes are independent and free-living in
1. mustard
2. castor
3. Pinus
4. Sphagnum
A human female with turner's syndrome
1. has 45 chromosomes with XO
2. has one additional X-chromosome
3. exhibits male characters
4. is able to produce children with normal husband
dB is a standard abbreviation used for the quantitative expression of
1. the density of bacteria in a medium
2. a particular pollutant
3. the dominant Bacillus in a culture
4. a certain pesticide
Which of the following occurs due to monosomy of sex chromosome?
1. Down's syndrome
2. Turner's syndrome
3. Haemophilia
4. Sickle cell anaemia
The step of regeneration of Phosphoenol pyruvate during C4 cycle occurs in:
| 1. | Only Mesophyll cells | 2. | Bundle Sheath cells |
| 3. | both a and b | 4. | Passage cells |
Which of the following statements about mycorrhizae is false?
| I. | In ectomycorrhizae, association between the fungus and plant is less intimate than in endomycorrhizae |
| II. | The mycorrhizal association of fungus and the plant may have had importance in the evolution of land plant |
| III. | The mycorrhizal association is a mutualistic symbiosis |
| IV. | Fungal partner is associated with the only roots of the higher plants (like angiosperms) |
| V. | Only advanced modern plants such as angiosperms possess mycorrhizae |
1. Only V
2. Only IV
3. Only I and III
4. Only II and V
The fact that the genetic code is almost universal in living organisms is considered to be evidence that all organisms:
(1) Are evolutionarily related
(2) Are genetically identical
(3) Have the same sequence of anticodons
(4) None of the above
Female mosquito
1. Needs our blood for reproduction
2. Parasite
3. Get some nutrition from our body
4. Both 1 and 2
Which of the following is not true about soil?
P. Soil not only supplies minerals but also harbours nitrogen fixing bacteria and other microbes
Q. It holds water
R. It supplies air to the roots
S. It acts as a matrix that stabilises the plant
T. It is a source of photosynthate
U. It is positively geotropic without any exception
1. S, T and U
2. T and U
3. S and U
4. R and T
How much amount of impurities are present in waste water which make it unfit for human use?
1. 0.01%
2. 0.1%
3. 0.2%
4. 2%
Water channels are made up of
1. Eight aquaporins
2. Eight different types of aquaporins
3 .Ten aquaporins
4. Ten different types of aquaporins
Which of the following character is common for all algal group.
1. Presence of cellulose in cell – wall.
2. Starch is reserve food material.
3. Presence of motile stage.
4. Only oogamous type of sexual reproduction.
State True (T) or False (F) for the following statements and select the correct option
A. Motor vehicles equipped with catalytic converter should use unleaded petrol because lead in the petrol inactivates the catalyst.
B. Recently government of India has instituted the Amrita Devi Bishnoi Wildlife Protection Award for
individuals from urban areas that have shown extraordinary courage and dedication in protecting
wildlife.
C. Reforestation may occur naturally in a deforested area.
A B C
1. T T F
2. T F T
3. T F F
4. F F T
What seems to be the most valid statement about root pressure?
1. It is the positive pressure responsible for transport of water most commonly.
2. It may provide a considerable push to the water column during transport.
3. It may help re establish the broken water columns during transpiration
4. Its effect is most observable during mid day
Pollen pistil interaction is
1. Chemically mediated process
2. Dynamic process
3. Genetically controlled process
4. More than one option is correct
Out of the following examples, how many are belonging
to Fabaceae, Solanaceae, Liliaceae?
Aloe, Indigofera, Asparagus, Colchicum, Belladonna,
Mulaithi
Fabaceae Solanaceae Liliaceae
1. 3 2 1
2. 2 2 2
3. 2 1 3
4. 4 1 1
Select the incorrect statement with respect to vessel
| 1. | Devoid of protoplasm. |
| 2. | Lignified wall. |
| 3. | Long cylindrical tube like cells. |
| 4. | Presence of vessel is characteristic of gymnosperm. |
What is correct?
| 1. | DNA content becomes double during G1- phase |
| 2. | Duration of interphase is short as compared to M-phase |
| 3. | G2-phase follows mitotic phase |
| 4. | DNA-replication occurs in S-phase |
The institute which encourages publication of local flora in India is
| 1. | NBRI | 2. | FRI |
| 3. | BSI | 4. | IARI |
The most important advantage of micropropagation is:
| 1. | production of virus free plants |
| 2. | rapid production of a large number of genetically identical superior plants |
| 3. | creation of somaclonal variations for effective germplasm collection |
| 4. | production of transgenic plants |
Members of the chrysophytes
1. Are macroscopic planktons
2. Are present in freshwater as well as in marine water
3. Have stiff cellulose plates on the outer surface
4. Cause PSP in human beings
Juvenile phase or vegetative phase is related with all, except
1. It is pre-reproductive phase in the life cycle of an individual
2. It is the period of growth
3. It is of different durations in different organisms
4. It involves the appearance of flowers in higher plants
How many microtubules are present in the axoneme part of eukaryotic flagellum?
1. 9
2. 27
3. 18
4. 20
Match correctly with respect to mode of propagation
1. Adventitious bud - Potato tuber
2. Offset - Lotus
3. Seed - Cereal
4. Rhizome - Terror of Bengal
Find odd one with respect to monoecious condition.
1. Chara
2. Marchantia
3. Cucurbits
4. Coconuts
Mark the incorrect match
1. Noise pollution - Dobson unit
2. national Forest Policy - 1988
3. Terror of Bengal - Eutrophic water bodies
4. Water (Prevention and Control of Pollution) Act - 1974
Which of the following component of phloem is mainly responsible for the storage of food material and other substances like resins, latex and mucilage?
1. Sieve tube
2. Companion cell
3. Bast fibres
4. Phloem parenchyma
Placentation in a syncarpous unilocular ovary where ovules occur on sutures is
1. Apical placentation
2. Parietal placentation
3. Marginal placentation
4. Superficial placentation
What was the cause of extinction of cichlid fish in lake Victoria in USA?
1. habitat loss and fragmentation
2. over-exploitation
3. alien species invasions
4. co-extinctions
The cultivation of hybrids is problematic. Which of the following is not one of them?
(1) They have to be produced every year.
(2) Progeny shows segregation and do not maintain hybrid characters.
(3) The productivity of hybrids is lower than that of the parents.
(4) Their production is expensive and thus costly to farmers.
|
Degenerate, triplet, commaless, overlapping, universal in eukaryotes only |
How many of the above features are associated with universal genetic code?
1. Five
2. Three
3. Four
4. Two
Which one of the following steps comes after electrophoresis in DNA finger printing?
1. Use of restriction endonuclease
2. Isolation of DNA
3. Southern blotting
4. DNA - RNA hybridization
| 1. | PS I | 2. | PS II |
| 3. | Both 1 and 2 | 4. | Neither 1 nor 2 |
Identify the correct set of statements with regard to the properties of humus.
| (a) | Highly resistant to microbial action |
| (b) | Dark-colored amorphous substance |
| (c) | End product of detritus food chain |
| (d) | Reservoir of nutrients |
| (e) | Undergoes decomposition very fast |
Choose the correct answer from the options given below:
1. (a), (b), and (d) only
2. (a),(b) and (e) only
3. (a) and (b) only
4. (b), (c), and (a)
| 1. | All members of Bacteria are single-celled and all members of Eukarya are multicellular |
| 2. | Only Eukaryotes have DNA |
| 3. | Only Eukaryotes have the ability to grow and reproduce |
| 4. | In Bacteria, there is an absence of membranous organelles, such as a nucleus |
| Statement A: | Amazon rain forest is called 'Lungs of planet' |
| Statement B: | Coevolved plant-pollinator mutualism does not result in extinction of one partner if the another is eliminated |
| 1. | Small plants | 2. | Fish |
| 3. | Man | 4. | Zooplankton |
| Assertion (A): | There is a need to standardise the naming of living organisms such that a particular organism is known by the same name all over the world |
| Reason (R): | Local names vary from place to place, which creates confusion |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
| Assertion (A): | Meiosis introduces new combination of traits in the progency. |
| Reason (R): | Meiosis involves crossing over. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
| 1. | Are very different from each other in their basic properties |
| 2. | Have morphological similarities |
| 3. | Can be placed in different families |
| 4. | Cannot be differentiated on the basis of their morphological features |
| 1. | All the steps in decomposition operate simultaneously on the humus. |
| 2. | Humus is dark colored amorphous substance |
| 3. | Humus undergoes decomposition at very slow rate |
| 4. | Humus serves as reservoir of nutrients. |
| Assertion (A): | Growth and differentiation in plants are closed. |
| Reason (R): | In plants, cells/tissues arising out of the same meristem have the same structures at maturity. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
| Assertion (A): | Tapetum is formed during the process of the formation of microsporangium. |
| Reason (R): | It plays an important role in guiding the pollen tube into the synergid. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Lack of relaxation between successive stimuli in sustained muscle contraction is known as
1. fatigue
2. tetanus
3. tonus
4. spasm
The eyes of Octopus and eyes of cat show different patterns of structure, yet they
perform similar function. This is an example of
1. homologous organs that have evolved due to convergent evolution
2. homologous organs that have evolved due to divergent evolution
3. analogous organs that have evolved due to convergent evolution
4. analogous organs that have evolved due to divergent evolution
What is the figure given below showing particular?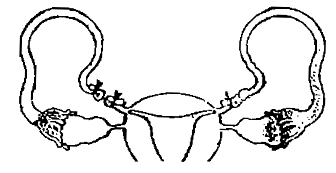
1. Ovarian cancer
2. Uterine cancer
3. Tubectomy
4. Vasectomy
Which of the following enzymes carries out the initial step in the digestion of milk in humans?
1. Rennin
2. Lipase
3. Trypsin
4. Pepsin
Hormones for the menstrual cycle are produced by
| 1. | ovaries only |
| 2. | uterus only |
| 3. | ovaries only |
| 4. | ovaries and anterior pitutory |
Which of the following epithelium is capable of forming ciliated epithelium by possessing cilia on its free surface?
(1) Compound
(2) Cuboidal
(3) Columnar and compound
(4) Cuboidal and columnar
Simple epithelium does not account for
| 1. | Made up of single layer of cells |
| 2. | Functions as lining of body cavities |
| 3. | Functions as lining of muscles |
| 4. | Is not protective in function |
Which is not associated with Mucosa?
(1) Villi, microvilli, goblet cells
(2) Gastric glands, intestinal crypts
(3) Lacteal, villi and microvilli
(4) Lacteal, duodenal glands
Which of the following is correct?
(1) Milk fats are digested by Rennin.
(2) A prolytic enzyme found in infants.
(3) Rennin is active at pH-1.8
(4) Lingual lipases are not present in gastric juice
Drug Absorption in the mouth involves
(1) Coming in contact of Mucosa of the mouth
(2) Coming in contact of upper surface of the tongue
(3) Coming in contact of palate of the mouth
(4) More than one of the above options are correct.
In the story of this world, evolution is a …... and in the story of life on Earth, evolution is……
1. Process, result of environmental changes
2. Result of environmental changes, process
3. Process, result of natural selection
4. Result of natural selection, process
The first heart sound is heard during the:
1. Beginning of ventricular systole
2. End of ventricular systole
3. Beginning of atrial systole
4. End of ventricular diastole
The Downstream processing occurs after
1. Biosynthetic phase
2. Separation phase
3. Purification phase
4. All of these
Which of the following option is completely permeable to water and impermeable to electrolytes?
1.Descending limb of the loop of Henle
2.Ascending limb of loop of Henle
3.Loop of Henle
4.DCT
Early diagnosis of a disease cannot be served by
| 1. | ELISA |
| 2. | PCR |
| 3. | Recombinant DNA Technology |
| 4. | X-Ray |
The polarity of the membrane at site A is reversed ie inside of the membrane becomes changed from negative to positive
1. Called as depolarization
2. Called as repolarization
3. Called as polarization
4. Called as hyperpolarization
The cerebral cortex is referred to
1. White matter
2. Grey matter
3. Granule matter
4. Pia mater
How many phosphodiesters bond are present in 5’ATTTTTGG 3’ sequence of nucleotides
(1)7
(2)9
(3)8
(4)6
Both the strands of the DNA are joined by
1.Disulphide bond
2.hydrogen bonds
3. Glycosidic bonds
4. phosphodiester bonds
The reflex arc ends at:
1. sensory neuron
2. motor neuron
3. effector
4. receptor
The hormone that acts on the exocrine pancreas and stimulates the secretion of water and bicarbonate ions is:
1. Gastrin
2. Secretin
3. Cholecystokinin
4. Gastric inhibitory peptide
Which of the following values is not equal? [Page 272]
1. Partial pressure of carbon dioxide in deoxygenated blood and partial pressure of carbon dioxide in tissues
2. Partial pressure of carbon dioxide in oxygenated blood and partial pressure of oxygen in tissues
3. Partial pressure of oxygen in deoxygenated blood and partial pressure of carbon dioxide in alveoli
4. Partial pressure of oxygen in alveoli and partial pressure of oxygen in oxygenated blood pumped by left ventricle
How many of the following hormones can change the chromosomal functions by binding with their receptors?
Cortisol, Iodothyronine, Testosterone, Estradiol, Insulin, melatonin
1. 6
2. 4
3. 5
4. 3
The condition of prolonged hyperglycemia leads to
| 1. | Diabetes Insipidus | 2. | Diabetes mellitus |
| 3. | Glycosuria | 4. | Both 2 and 3 |
When blood levels of carbon dioxide rise, the rate and depth of breathing _________.
1. decreases
2. increases
3. stays the same
4. stops
Which of the following set of animals belong the phylum hemichordate?
1. Balanoglossus, Saccoglossus
2. Salpa, Doliolum
3. Petromyzon, Myxine
4. Dentalium, Chaetopleura
During micturition , the contraction of detrusor muscles of urinary bladder is under the control of
1. Sympathetic nerve
2. Parasympathetic nerve
3. Pudendal nerve upon stimulation
4. Pudendal nerve upon inhibition
Areolar tissue contains secretory cells which secrete the fibres and other components of matrix. Such cells are
| 1. | Fibroblasts | 2. | Macrophages |
| 3. | Mast cells | 4. | Fibrocytes |
Arrange the geological periods in ascending order jurassic, permian, Carboniferous, Devonian, Cretaceous, Triassic
1 Devonian, Carboniferous, Permian, Jurassic, Triassic, Cretaceous
2 Devonian, Permian, Carboniferous, Triassic, Jurassic, Cretaceous
3 Carboniferous, Devonian, Permian, Triassic, Jurassic, Cretaceous
4 Devonian, Carboniferous, Permian, Triassic, Jurassic, Cretaceous
Read the following paragraph with blanks A, B, C, and D.
Hugo de Vries based his work on A brought forth the idea of mutations. Mutations are random and B while Darwinian variations are small and C . Evolution for Darwin was gradual while de Vries believed mutation caused speciation and hence called it D.
Choose the option which correctly fills in the blanks A, B, C, and D.
1 A - Evening primrose
B -Directional
C - Directionless
D - Saltation
2 A - Evening primrose
B - Directional
C - Directionless
D - Natural selection
3 A - Garden pea
B - Directionless
C - Directional
D - Saltation
4 A - Evening primrose
B -Directionless
C - Directional
D - Saltation
Placoid scales, ventral mouth, heterocercal caudal fin and internal fertilization are the characteristics of
1. Scoliodon
2. Anguilla
3. Gambusia
4. Exocoetus
Which of the following is a mismatch?
| 1. | Amphibia | : Moist skin without scales, eyes have eyelids, sexes are separate and external fertilization |
| 2. | Osteichthyes | : 4 pair of gills; skin covered with cycloid/ctenoid scales, air bladder is present |
| 3. | Cyclostomata | : Elongated body bearing 6-15 pairs of gill slits, closed type circulation; have paired fins |
| 4. | Reptilia | : Body covered by dry and cornified scales, internal fertilization and direct development |
Cirrhosis of liver is caused by
1 Cocaine
2 Alcohol
3 LSD
4 Morphine
Which of the following is associated with a decrease in cardiac output?
(1) Sympathetic nerves
(2) Parasympathetic neural signals
(3) Pneumotaxic center
(4) Adrenal medullary hormones
Tick mark the incorrect statement about adult haemoglobin
1. it is made up of four sub-units
2. Two sub-units are of -type and two sub-units of -type
3. It has quartemary structure of protein
4. It is a simple protein
DNA can be visualized through UV rays if it is stained with:
| 1. | Ethidium bromide | 2. | Polyethylene glycol |
| 3. | Tritiated thymidine | 4. | Colchicine |
Under normal physiological conditions in human being every 100 ml of oxygenated blood can deliver _____________ml of O2 to the tissues.
1. 10 ml
2. 2 ml
3. 5 ml
4. 4 ml
| Statement I: | Proteins encoded by genes crylAc and cryllAb control corn borer, that of crylAb control the cotton bollworms |
| Statement II: | Bt toxin protein exist as inactive protoxins but once an insect ingest the inactive toxin, it is converted into an active form of toxin due to the alkaline pH of the gut |
| 1. | Both Statement I and Statement II are true |
| 2. | Both Statement I and Statement II are false |
| 3. | Statement I is true but Statement II is false |
| 4. | Statement I is false but Statement II is true |
| Assertion: | At the start of oogenesis, there are few million oogonia in each ovary and at puberty only 60,000 - 80,000 primary follicles are left in each ovary |
| Reason: | There is degeneration of the primary follicles from birth to puberty by follicular atresia. |
| 1. | Both assertion and reason are true and the reason is the correct explanation of the assertion. |
| 2. | Both assertion and reason are true and the reason is not the correct explanation of the assertion. |
| 3. | Assertion is true but reason is false. |
| 4. | Both assertion and reason are false. |
| Assertion (A): | In humans, leucocytes are only nucleated blood cells |
| Reason (R): | Monocytes are largest and most abundant type of leucocytes |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
| Assertion (A): | Adolescence is a very vulnerable phase of mental and psychological development of an individual. |
| Reason (R): | Addiction to drugs or alcohol is very common during adolescence. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
| Assertion(A): | With time, there is greater intake and addiction of drugs among addicts. |
| Reason(R): | With repeated use of drugs, tolerance level of receptors declines. |
| 1. | Both (A) and (R)are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true and (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
| 1. | Spermicidal action |
| 2. | Inhibit the release of gonadotropins |
| 3. | Alter the quality of cervical musuc |
| 4. | Inhibition of ovulation |
In which of the following case reaction goes farthest to completion?
1. K=103
2. K=10-2
3. K=10
4. K=100
The products of the electrolysis of aqueous solution of common salt are:
1. Na + Cl2
2. H2 + O2
3. NaOH + H2 + Cl2
4. NaOH + Cl2 + O2
XY. Y is:
1.
2.
3.
4.
The electric charge for electrode deposition of 1g equivalent of a substance is:
1. 1 ampere per second
2. 96,500 coulomb per second
3. 1 ampere for 1 hour
4. charge on 1 mole of electrons
For the reaction 2NO2 + F2 → 2NO2F, following
mechanism has been provided,
NO2 + F2 NO2F+F
NO2 + F NO2F
Thus, rate expression of the above
reaction can be written as:
1. r = K[NO2]2[F2]
2. r = K[NO2 ][F2]
3. r = K[NO2]
4. r = K[F2]
Identify (Z) in the following reaction reaction series,
C2H5I (X) (Y) (Z):
1. CH3-CH2-CN
2.
3.
4.
The compound that generate methyl ethyl ketone upon oxidation is -
1. 2-Propanol
2. 1-Butanol
3. 2-Butanol
4. tert-Butyl alcohol
What is the end product in the following sequences of operations?
Acetamide
1. CH3NH2
2. C2H5NH2
3. CH3CN
4. CH3COONH4
Alkanamide that gives 1-phenylethylamine on Hoffmann's reaction is:
1. 2-Phenylpropanamide
2. 3-Phenylpropanamide
3. 2-Phenylethanamide
4. N-Phenylethanamide
Colloidal solution of gold prepared by different methods of different colours because of:
1. different diameters of colloidal gold particles
2. variable valency of gold
3. different concentration of gold particles
4. impurities produced by different methods
Which of the following is used to make non stick cookware ?
1. PVC
2. Polysterene
3. Polyethylene
4. PTFE
The pH of a solution obtained by mixing 100ml of 0.2 M CH3COOH with 100ml of 0.2 M NaOH would be (given pKa for CH3COOH = 4.74)
1. 4.74
2. 8.87
3. 9.10
4. 8.57
Propene can be converted into 1-propanol by oxidation. Indicate which of reagents amongst the following is ideal to effect the above conversion?
1.
2.
3.
4.
Ordinarily the barrier to rotation about a carbon-carbon double bond is quite high but in compound P double bond between two rings was observed by NMR to have a rotational energy barrier of only about 20 cal.\mol., showing that it has lot of single bond charcter. The reason for this is
(1) Double bond having partial triple bond charcter because of resonance
(2) Doule bond undergo flipping
(3) Double bond having very high single bond charcter because of aromaticity gained in both three and five membered rings.
(4) +I effect of nC3H7 groups makes double bond having partial single bond character.
Acid strength of the conjugate acids of the following are-
(1) I > II > III > IV
(2) III > II > I > IV
(3) IV > III > II > I
(4) None of these
Atomic radius of silver is 144.5 pm. The unit cell of silver is a face centred cube. Calculate the density of silver.
(1) 10.50 g/cm3
(2) 16.50 g/cm3
(3) 12.30 g/cm3
(4) 15.50 g/cm3
Which of the following is not a chromophore?
(1) —N=N—
(2) —NO
(3) —NO2
(4) —NH2
Methane reacts with conc. HNO3 at high temperature to yield:
(1) CO2 and H2O
(2) HCHO
(3) HCOOH
(4) CH3NO2
Which of the following is an example of the elimination reaction?
1. Chlorination of methane
2. Dehydration of ethanol
3. Nitration of benzene
4. Hydroxylation of ethylene
Which of the statements is not true?
1. On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed
2. Na2Cr2O7 is preferred over K2Cr2O7 in volumteric analysis
3. K2Cr2O7 solution in acidic medium is orange
4. K2Cr2O7 solution becomes yellow on increasing the pH beyond 7
Amount of oxalic acid in grams that is required to obtain 250 ml of a semi-molar solution is
(1) 17.25 g
(2) 17.00 g
(3) 15.75 g
(4) 15.00 g
Acetic acid has Ka = 1.8 × 10–5 while formic acid had Ka = 2.1 × 10–4. What would be the magnitude of the emf of the cell
Pt(H2) Pt(H2) at 25°C
(1) 0.0315 volt
(2) 0.0629 volt
(3) 0.0455 volt
(4) 0.0545 volt
What is the equivalent mass of when it is converted into I2 in acid medium
(1) M/6
(2) M/7
(3) M/5
(4) M/4
Give the correct order of initials T or F for following statements. Use T if statement is true and F if it is false.
(i) In Gold schmidt thermite process aluminium acts as a reducing agent.
(ii) Mg is extracted by electrolysis of aq. solution of
(iii) Extraction of Pb is possible by carbon reduction method
(iv) Red Bauxite is purified by Serpeck's process
(1) TTTF
(2) TFFT
(3)FTTT
(4) TFTF
The decreasing values of bond angles from (106°) to (91°) down group-15 of the periodic table is due to
(1) decreasing lp – bp repulsion
(2) increasing electronegativity
(3) increasing bp – bp repulsion
(4) increasing p-orbital character in
The oxidation states of the S atom in from left to right, respectively, are:
1. + 6, 0, 0, and +6
2. +5, +1, +1, and +5
3. +5, 0, 0, and +5
4. +3, +1, +1, and +3
The correct increasing order of the acidity of is
(1)
(2)
(3)
(4)
The inversion of cane sugar proceeds with half life of 500 minute at pH 5 for any concentration of sugar. However if pH = 6, the half life changes to 50 minute. The rate law expression for the sugar inversion can be written as
(1)
(2)
(3)
(4)
For \(A \rightarrow B\), \(\Delta H = 4\ kcal\ mol^{-1}\), \(\Delta S = 10\ cal\ mol^{-1}\ K^{-1}\), the reaction is spontaneous when the temperature is:
1. 400 K
2. 300 K
3. 500 K
4. None of the above
The compound that is most susceptible to a nucleophilic attack in the carbonyl group is:
1.
2.
3.
4.
For any H-like system, the ratio of velocities of I, II & III orbit i.e., will be:
1. 1 : 2 : 3
2. 1 : 1/2 : 1/3
3. 3 : 2 : 1
4. 1 : 1 : 1
Order of nucleophilicity in polar aprotic solvent?
1.
2.
3.
4.
Arrange the reactivity of given compounds in decreasing order for electrophilic addition reaction:
(i)
(ii)
(iii)
(iv)
Select the correct answer from the codes given below:
Codes:
(1) (iv) (i) (ii) (iii)
(2) (iii) (ii) (i) (iv)
(3) (ii) (iii) (i) (iv)
(4) (ii) (iii) (iv) (i)
A Planar Complex (Mabcd) gives –
1. Two Optical isomer
2. Two geometrical isomer
3. Three optical isomer
4. Three geometrical isomers
Nitrogen show different oxidation states in the range :
1. 0 to +5
2. -3 to +5
3. -5 to +3
4. -3 to +3
A biosphere is composed of
1. Living organisms
2. Living organisms + Lithosphere
3. Living organisms + lithosphere + atmosphere
4. Living organisms + lithosphere + atmosphere + Hydrosphere
-helix is also known as:
1. Sheet structure
2. Flat structure
3. 3.613 helix
4. 4.915 helix
| Molar mass | |
| 461 |
Which among the following is not an oxide ore?
1. Malachite
2. Cuprite
3. Zincite
4. Bauxite
Under hydrolytic conditions, the compounds used for the preparation of the linear polymer and for chain termination, respectively, are
1.
2.
3.
4.
Which of the following pairs has the most significant size variation?
| 1. | Na and Na+ | 2. | Na and K |
| 3. | Cl and Cl– | 4. | Mg and Mg+ |
Match the soaps given in Column I with items given in Column II.
Column I Column II
1. Soap chips (a) Dried miniature soap bubbles
2. Soap granules (b) Small broken pieces of soap formed from melted soaps
3. Soap powder (c) Soap powder + abrasives + builders
4. Scouring soap (d) Soap powder + builders like
1. (i) → (a) (ii) → (b) (iii) → (d) (iv) → (c)
2. (i) → (d) (ii) → (a) (iii) → (b) (iv) → (c)
3. (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
4. (i) → (c) (ii) → (a) (iii) → (d) (iv) → (b)
Which one is communicable disease?
(1) Scurvy
(2) Diabetes
(3) Beri-beri
(4) Cholera
The inside of a cell is a
1. reducing medium
2. oxidising medium
3. neutral medium
4. None of these
The volume occupied by 9.0 g of nitrogen gas at 300 K and 750 mmHg pressure is
(1) 5.854
(2) 6.7432
(3) 8.8462
(4) 8.0225
Ebonite is
(1) Natural rubber
(2) Synthetic rubber
(3) Highly vulcanized rubber
(4) Polypropene
The free energy change is negative when -
1. The surroundings do no electrical work on the system.
2. The surroundings do electrical work on the system.
3. The system does electrical work on the surroundings.
4. The system does no electrical work on the surroundings.
Photochemical smog occurs in warm, dry, and sunny climates. One of the following is not amongst the components of photochemical smog. Identify it:
1. NO2
2. O3
3. SO2
4. Unsaturated hydrocarbon
Given below are two statements:
| Assertion (A): | Greenhouse effect was observed in houses used to grow plants and these are made of green glass. |
| Reason (R): | Greenhouse name has been given because glasshouses are made of green glass. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
A force \(- F \hat k\) acts on point \(O\) the origin of the coordinate system. What is the torque about the point \((1,-1)\)?
1. \(-F(\hat i + \hat j)\)
2. \(F(\hat i + \hat j)\)
3. \(-F(\hat i -\hat j)\)
4. \(F(\hat i - \hat j)\)
The centre of the mass of \(3\) particles, \(10~\text{kg},\) \(20~\text{kg},\) and \(30~\text{kg},\) is at \((0,0,0).\) Where should a particle with a mass of \(40~\text{kg}\) be placed so that its combined centre of mass is \((3,3,3)?\)
1. \((0,0,0)\)
2. \((7.5, 7.5, 7.5)\)
3. \((1,2,3)\)
4. \((4,4,4)\)
The ionisation potential of hydrogen atom is
1. 13.60 volt
2. 8.24 volt
3. 10.36 volt
4. 14.24 bolt
The resistance of platinum wire at 0 is 22.05 and at 100 it becomes 22.70 . When the wire is heated to a temperature of t the resistance of wire becomes 24.91. The value of t is
1. 220
2. 440
3. 310
4. 550
Two waves of intensity ratio 9:1 interfere to produce fringes in a young's double-slit experiment, the ratio of intensity at maxima to the intensity at minima is
1. 4:1
2. 9:1
3. 81:1
4. 9:4
How much energy is contained in a particle that has a mass of
The moment of inertia of pulley is I and its radius is R. The string doesn't slips on the pulley, as system is released, the acceleration of the system is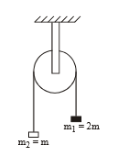 \(\)
\(\)
1.
2.
3.
4.
A physical parameter '\(a\)' can be determined by measuring the parameters \(b\), \(c\), \(d\) and \(e\) using the relation, \(a= \dfrac{b^{\alpha}c^{\beta}}{d^{\gamma}e^{\delta}}.\) If the maximum errors in the measurement of \(b, ~c, ~d,~\text{and}~e\) are \(b_1\%,~c_1\%,~d_1\%~\text{and}~e_1\%\), then the maximum error in the value of '\(a\)' determined by the experiment is:
1. \((b_1+c_1+d_1+e_1)\%\)
2. \((b_1+c_1-d_1-e_1)\%\)
3. \((\alpha b_1+\beta c_1-\gamma d_1-\delta e_1)\%\)
4. \((\alpha b_1+\beta c_1+\gamma d_1+\delta e_1)\%\)
Which of the following pairs is wrong?
1. Pressure-Baromter
2. Relative density-Pyrometer
3. Temperature-Thermometer
4. Earthquake-Seismograph
A particle travels 10 m in first 5 sec and 10m in the next 3 sec. Assuming constant acceleration what is the distance travelled in next 2 sec ?
1. 8.3 m
2. 9.3 m
3. 10.3 m
4. None of above
A coin, placed on a rotating turn-table slips, when it is placed at a distance of 9 cm from the centre. If the angular velocity of the turn-table is trippled, it will just slip, if its distance from the centre is
1. 27 cm
2. 9 cm
3. 3 cm
4. 1 cm
A body of mass 5 kg is suspended by a spring balance on a smooth inclined plane as shown in the figure. The spring balance measure:
1. 50 N
2. 25 N
3. 500 N
4. 10 N
A transformer is employed to reduce 220 V to 11 V. The primary draws a current of 5 A and the secondary 90 A. The efficiency of the transformer is
1. 20%
2. 40%
3. 70%
4. 90%
The electric field between the plates of a parallel plate capacitor when connected to a certain battery is E0. If the space between the plates of the capacitor is filled by introducing a material of dielectric constant K without disturbing the battery connections, the field between the plates shall be
1. K E0
2. E0
3.
4. None of the above
Which of the following are not electromagnetic waves
1. Cosmic rays
2. Gamma rays
3. -rays
4. X-rays
If the earth suddenly shrinks (without changing mass) to half of its present radius, the acceleration due to gravity will be:
1. g/2
2. 4g
3. g/4
4. 2g
Which of the following quantities does not depend upon the orbital radius of the satellite ?
1.
2.
3.
4.
Which of the following combinations should be selected for better tuning of an L-C-R circuit used for communication?
1.
2.
3.
4.
A circuit contains an ammeter, a battery of 30 V and a resistance 40.8Ω all connected in series. If the ammeter has a coil of resistance 480Ω and a shunt of 20Ω then reading in the ammeter will be :
1. 0.5A
2. 0.25A
3. 2A
4. 1A
A bar magnet of length L and magnetic dipole moment M is bent in the form of an are as shown in figure. The new magnetic dipole moment will be
1. M
2. 3M/π
3. 2/πM
4. M/2
The deformation of a wire under its own weight compared to the deformation of the same wire subjected a tension equal to the weight of the wire is
1. same
2. one third
3. half
4. one fourth
A tank is filled with water up to a height H. Water is allowed to come out of a hole P in one of the walls at a depth D below the surface of water. Express the horizontal distance x in terms of H and D
1.
2.
3.
4.
In the Young's double slit experiment, if the phase difference between the two waves interfering at a point is ϕ, the intensity at that point can be expressed by the expression-
(where A and B depend upon the amplitudes of the two waves)
(1)
(2)
(3)
(4)
The phase difference between two points separated by 1m in a wave of frequency 120 Hz is 90°. The wave velocity is :
1. 180 m/s
2. 240 m/s
3. 480 m/s
4. 720 m/s
In the circuit given below, V(t) is the sinusoidal voltage source, voltage drop (t) across the resistance R is
(1) Is half wave rectified
(2) Is full wave rectified
(3) Has the same peak value in the positive and negative half cycles
(4) Has different peak values during positive and negative half cycle
The wavelength of de-Broglie wave is 2m, then its momentum is (h = J-s)
(a) kg-m/s (b) kg-m/s
(c) kg-m/s (d) kg-m/s
In heat transfer, which method is based on gravitation ?
(1) Natural convection
(2) Conduction
(3) Radiation
(4) Stirring of liquids
In a reversible isochoric change -
1. ΔW = 0
2. ΔQ = 0
3. ΔT = 0
4. ΔU = 0
| 1. | \(\dfrac{25}{9}\) | 2. | \(\dfrac{17}{6}\) |
| 3. | \(\dfrac{9}{5}\) | 4. | \(\dfrac{4}{3}\) |
Radius of the first orbit of the electron in a hydrogen atom is 0.53 Å. So, the radius of the third orbit will be
(1) 2.12 Å
(2) 4.77 Å
(3) 1.06 Å
(4) 1.59 Å
Uranium ores contain one radium-226 atom for every 2.8 x Uranium-238 atoms. Calculate the half-life of given that the half-life of is 1600 years and is a decay product of .
(1) 1.75 x years
(2) 1600 x years
(3) 4.5 x years
(4) 1600 x years
Binding energy per nucleon plot against the mass number for stable nuclei is shown in the figure. Which curve is correct ?
1. A
2. B
3. C
4. D
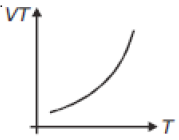
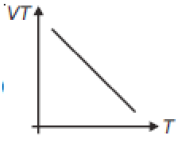
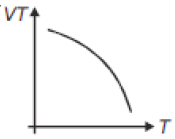
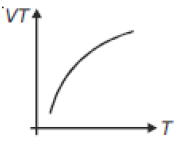
If heat energy is given to an ideal gas at constant pressure, then select hte graph which best represents the variation of VT with temperature (T).
1. 
2. 
3. 
4. 
In the given figure
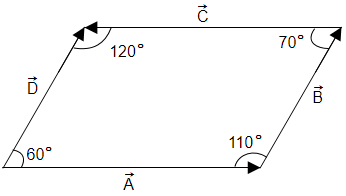
| 1. | Angle between \(\overrightarrow {A}\) and \(\overrightarrow {B}\) is \(110^{\circ}\) |
| 2. | Angle between \(\overrightarrow {C}\) and \(\overrightarrow {D}\) is \(60^{\circ}\) |
| 3. | Angle between \(\overrightarrow {B}\) and \(\overrightarrow {C}\) is \(110^{\circ}\) |
| 4. | Angle between \(\overrightarrow {B}\) and \(\overrightarrow {C}\) is \(70^{\circ}\) |
Two forces, and are acting on a body. One force is doubled of the other force and the resultant is equal to the greater force. Then the angle between the two forces is -
(A)
(B)
(C)
(D)
If represent the permittivity and permeability of vacuum and represent the permittivity and permeability of the medium, then refractive index of the medium is given by :
(1) (2)
(3) (4)
In Bohr’s model, the atomic radius of the first orbit is r0 ; then the radius of the third orbit is
1. r0/9
2. r0
3. 9r0
4. 3r0
Two ideal diodes are connected to a battery as shown in the circuit. The current supplied by the battery is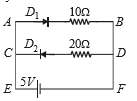
1. 0.75 A
2. 0.5 A
3. 0.25 A
4. zero

If the wavelength of a ray of light from a source of lights is 3000 in air, then the wavelength of that ray of light in a liquid in which S is placed will be: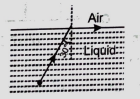
(1) 4000
(2) 1500
(3) 9000
(4) 3500
Select the correct option.
(1) The velocity of light is constant in all media
(2) A convex lens always forms a real image
(3) If the aperture of the lens is halved then image size will be halved
(4) The ray optics is applicable when the size of the obstacle is more than the wavelength of light
Two Carnot engines x and y are working between the same source temperature \(T_1\) and the same sink temperature \(T_2\). If the temperature of the source in Carnot engine x is increased by \(\Delta T\), and in the Carnot engine y, the temperature of the sink is increased by\(\Delta T\), then the efficiency of x and y becomes \(\eta_\mathrm x\) and\(\eta_\mathrm y\). Then:
| 1. | \(\eta_{\mathrm{x}}=\eta_{\mathrm{y}}\) |
| 2. | \(\eta_{\mathrm{x}}<\eta_{\mathrm{y}}\) |
| 3. | \(\eta_{\mathrm{x}}>\eta_{\mathrm{y}}\) |
| 4. | The relation between \(\eta_{\mathrm{x}}\) and \(\eta_{\mathrm{y}}\) depends on the nature of the working substance |
| 1. | the gravity of the earth | 2. | the mass of the block |
| 3. | spring constant | 4. | both (2) & (3) |
Two bar magnets are held together tightly in a vibration magnetometer. When their like poles are together, they make \(20\) oscillations per minute and when their unlike poles are together, they make \(8\) oscillations per minute. The ratio of the magnetic dipole moments of two bar magnets is:
1. \(29:21\)
2. \(6:15\)
3. \(1:6\)
4. \(25:4\)
In the circuit shown, X is joining to Y for a long time, and then X is joined to Z. The total heat produced in is ( symbols have their usual meaning)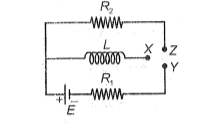
The principle of conservation of energy and conservation of mechanical energy applicable respectively for
(1) Conservative and non-conservative forces
(2) Conservative and conservative force
(3) Non-conservative and conservative forces
(4) All forces and conservative forces
A man can row a boat with \(8\) km/h in still water. He is crossing a river of width \(8\) km, where the speed of water flow is \(4\) km/h. What direction should he head the boat to cross the river in the shortest time?
1. \(30^{\circ}\) with the current
2. \(60^{\circ}\) with the current
3. \(90^{\circ}\) with the current
4. \(120^{\circ}\) with the current
A ball is dropped from a bridge of 122.5 metre above a river. After the ball has been falling for two seconds, a second ball is thrown straight down after it. Initial velocity of the second ball so that both hit the water at the same time is
(1) 49 m/s
(2) 55.5 m/s
(3) 26.1 m/s
(4) 9.8 m/s