Botany - Section A
1. Identify the incorrect statement:
| 1. |
Thylacine is an extinct carnivorous marsupial that was native to the Australian mainland. |
| 2. |
The mass extinction currently in progress is the fifth mass extinction. |
| 3. |
Habitat loss and fragmentation is the most important cause driving animals and plants to extinction. |
| 4. |
The Nile perch introduced into Lake Victoria became invasive. |
2. Filiform apparatus is a part of:
| 1. |
synergids at the chalazal end of the female gametophyte |
| 2. |
antipodals at the chalazal end of the female gametophyte |
| 3. |
synergids at the micropylar end of the female gametophyte |
| 4. |
antipodals at the micropylar end of the female gametophyte |
3. Temperature is the most important environmental factor for organisms because:
| 1. |
it affects enzyme kinetics |
| 2. |
its availability is closely linked to the availability of light |
| 3. |
it causes more water to be available in oceans |
| 4. |
vast majority of organisms are eurythermals |
4. Montreal protocol:
| 1. |
is an international treaty designed to protect the ozone layer signed in 1987, and entered into force in 1989. |
| 2. |
is an international treaty designed to control global warming signed in 1991, and entered into force in 1993. |
| 3. |
is an international treaty designed to protect the ecological hotspots of biodiversity signed in 1987, and entered into force in 1989. |
| 4. |
is an international treaty designed to protect wetlands signed in 1985, and entered into force in 1987. |
5. Who proposed the five kingdom classification?
1. Carolus Linnaeus
2. Aristotle
3. Bentham and Hooker
4. R. H. Whittaker
6. A heterogeneous collection of fructose polymers, produced by many types of plants, is:
1. Lectin
2. Ricin
3. Inulin
4. Concanavalin
7. Identify the incorrect statement:
| 1. |
Pollen sacs are developed microsporangia |
| 2. |
Pollen grains are developed microspores |
| 3. |
Vegetative cell of the pollen grain is bigger than the generative cell |
| 4. |
In over 60 percent of angiosperms pollens are shed at 3-celled stage |
8. Alnus can grow in nutrient-poor soils, where few other trees thrive, because of its association with nitrogen fixing bacterium:
1. Anabaena
2. Rhizobium
3. Azotobacter
4. Frankia
9. Calotropis are avoided by grazing herbivores because they produce:
| 1. |
stipules |
2. |
thorns |
| 3. |
mustard oils |
4. |
cardiac glycosides |
10. A double-stranded DNA molecule globally has percentage base pair equality: A% = T% and G% = C%. One such DNA molecule is found to have 23 % Adenine. What is the expected % of Uracil in this DNA?
11. A diagram of the transverse section of a monocot leaf is given below with certain parts indicated as letters of the alphabet. Select the option which incorrectly matches the alphabet with the part it represents:

| 1. |
A - Adaxial epidermis |
2. |
B - Phloem |
| 3. |
C - Mesophyll |
4. |
D - Abaxial epidermis |
12. Eicchornia crassipes,
Parthenium hysterophorus, and
Lantana camara are:
| 1. |
native species of India |
| 2. |
critically endangered plants of India |
| 3. |
invasive plant species |
| 4. |
medicinal plants |
13. Identify the correctly matched pair:
|
Pteriodophyte |
Class |
| 1. |
Psilopsida |
Adiantum |
| 2. |
Lycopsida |
Selaginella |
| 3. |
Sphenopsida |
Dryopteris |
| 4. |
Pteropsida |
Equisetum |
14. Identify the incorrect comparison between cellulose and starch:
|
|
Cellulose |
Starch |
| 1. |
Monomer subunit |
Beta Glucose |
Alpha Glucose |
| 2. |
Helical secondary structure |
Yes |
No |
| 3. |
Linkage |
β(1→4)-glycosidic bonds |
α(1→4)-glycosidic bonds |
| 4. |
Type |
Homopolymer |
Homopolymer |
15. Between Telophase I and Prophase II:
| 1. |
The nuclear membrane disappears |
| 2. |
DNA does not replicate |
| 3. |
A tetrad of cells is formed |
| 4. |
There is a long lasting resting stage |
16. Which of the following statements is correct regarding diffusion:
| 1. |
Diffusion of solids rather than diffusion in solids is more likely. |
| 2. |
The diffusion rate depends upon the size of the substances; smaller substances diffuse slower |
| 3. |
Diffusion is dependent on a living system |
| 4. |
Movement by diffusion is passive and no energy expenditure takes place in the process |
17. In which of the following plants, the flowers are epigynous and the position of ovary is described as inferior?
| 1. |
Mustard |
2. |
Rose |
| 3. |
China rose |
4. |
Ray florets of sunflower |
18. Colchicum autumnale:
1. is non-endospermous
2. has dithecous anther
3. has reticulate venation
4. exhibits axile placentation
19. The most commonly used biological pesticide worldwide is:
1. Agrobacterium tumefaciens
2. Pseudomonas putida
3. Bacillus thuringiensis
4. Trichoderma harzianum
20. The type of meristem that may have evolved in grasses in response to damage by grazing herbivores will be:
1. Shoot apical meristem
2. Root apical meristem
3. Intercalary meristem
4. Floral meristem
21. Regarding RuBisCO:
| I: |
It is the most abundant protein in the whole of the biosphere. |
| II: |
Mg2+ is its activator |
| 1. |
Only I is correct |
| 2. |
Only II is correct |
| 3. |
Both I and II are correct |
| 4. |
Both I and II are incorrect |
22. Which of the following does not take place during diakinesis of Meiosis I?
| 1. |
Disappearance of nucleolus |
| 2. |
Decondensation of chromosomes |
| 3. |
Nuclear envelope breaks down |
| 4. |
Assembly of meiotic spindle |
23. Identify the incorrect statement regarding
Bacillus thuringiensis?
| 1. |
forms protein crystals during a particular phase of their growth which contains a toxic insecticidal protein. |
| 2. |
The Bt toxin protein exists as an inactive protoxin that is converted into an active form by an alkaline pH in the insect's gut. |
| 3. |
Activated toxin binds to the surface of midgut epithelial cells, creates pores, causes cell swelling and lysis and eventually death of the insect. |
| 4. |
Proteins encoded by the genes cryIAc and cryIIAb control the corn borer, that of cryIAb controls cotton bollworms. |
24. Plant experimentally grown in a growth medium die when the medium lacks boron but does well when boron is added at a low concentration of 5 ppm in the medium. Boron should thus be classified as:
1. An essential macronutrient
2. A non-essential macronutrient
3. An essential micronutrient
4. A non-essential micronutrient
25. Consider the following two statements:
| I: |
Enzyme catalyzed reactions are affected by the change in the temperature and pH. |
| II: |
Change in pH and temperature affects the tertiary structure of proteins. |
1. Both
I and
II are correct and
II explains
I
2. Both
I and
II are correct but
II does not explain
I
3.
I is correct but
II is incorrect
4.
I is incorrect but
II is correct
26. Match each item in Column I with the one in Column II and select the correct match from the codes given:
|
COLUMN I
[PGR] |
|
COLUMN II
[Discovered by] |
| A |
Auxin |
P |
F. W. Went |
| B |
Gibberellic acid |
Q |
E. Kurosawa |
| C |
Kinetin |
R |
Miller et al. |
| D |
Ethylene |
S |
H. H, Cousins |
Codes:
|
A |
B |
C |
D |
| 1. |
P |
Q |
R |
S |
| 2. |
Q |
P |
S |
R |
| 3. |
R |
S |
Q |
P |
| 4. |
S |
R |
P |
Q |
27. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
COLUMN I |
|
COLUMN II |
| A |
Standing crop |
P |
Mass of living material at each trophic level at a particular time |
| B |
Standing state |
Q |
The amount of nutrients present in the soil at any given time |
| C |
Net primary productivity |
R |
The rate of formation of new organic matter by consumers |
| D |
Secondary productivity |
S |
Available biomass for the consumption to heterotrophs |
Codes:
|
A |
B |
C |
D |
| 1. |
S |
P |
Q |
R |
| 2. |
P |
Q |
R |
S |
| 3. |
P |
Q |
S |
R |
| 4. |
R |
P |
Q |
S |
28. The Ti Plasmid [ having T-DNA] is considered a natural genetic engineer and can transform:
1. bacterial cells
2. dicot plant cells
3. monocot plant cells
4. animal cells
29. Identify the incorrect comparison between monocots and dicots in general:
|
Character |
Monocots |
Dicots |
| 1. |
Leaves |
Parallel venation |
Reticulate venation |
| 2. |
Roots |
Primary root of short duration, replaced by adventitial roots forming fibrous or fleshy root systems |
Develops from the radicle. Primary root often persists forming strong tap roots and secondary roots |
| 3. |
Plant stem: Vascular bundles |
Ring of primary bundles with cambium, differentiated into cortex and stele |
Numerous scattered bundles in ground parenchyma, cambium mostly absent, no differentiation between cortical and stelar regions |
| 4. |
Flowers |
Parts in threes (trimerous) or multiples of three |
Fours (tetramerous) or fives (pentamerous) |
30. Identify the correct statements:
| I: |
Primary succession is faster than the secondary succession |
| II: |
Both hydrarch and xerarch successions lead to mesic conditions |
| III: |
Rooted submerged plants are pioneers in primary hydrarch succession |
1. Only II
2. Only III
3. Only II and III
4. Only I
31. Which of the following statement is true about the difference between
Cycas and
Pinus?
| 1. |
Pinus has coralloid roots associated with N2-fixing cyanobacteria, whereas Cycas has roots with fungal association in the form of mycorrhiza |
| 2. |
Cycas is heterosporous, whereas Pinus is homosporous |
| 3. |
Male cones and female megasporophylls are borne on different trees in Cycas, whereas they are borne on the same tree in Pinus |
| 4. |
Stems of Cycas are branched, whereas Pinus has unbranched stems |
32. Consider the given two statements:
| I: |
The oxygenation reaction of RuBisCO is a wasteful process. |
| II: |
3-phosphoglycerate is not created. |
| 1. |
Both I and II are correct and II explains I |
| 2. |
I is correct but II is incorrect |
| 3. |
Both I and II are correct but II does not explain I |
| 4. |
I is incorrect but II is correct |
33. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
Group of protozoans |
|
Example |
| A. |
Amoeboid |
P. |
Entamoeba |
| B. |
Flagellated |
Q. |
Paramecium |
| C. |
Ciliated |
R. |
Trypanosoma |
| D. |
Sporozoan |
S. |
Plasmodium |
Codes:
|
A |
B |
C |
D |
| 1. |
Q |
S |
P |
R |
| 2. |
S |
Q |
R |
P |
| 3. |
P |
R |
Q |
S |
| 4. |
R |
P |
S |
Q |
34. Identify the correct statements:
| I: |
Kalyan sona and Sonalika are semi-dwarf varieties of wheat. |
| II: |
aya and Ratna are varieties of rice developed in India. |
| III: |
Saccharum barberi had thicker stems and higher sugar content and did not grow well in North India |
| 1. |
Only II |
| 2. |
Only I and II |
| 3. |
Only II and III |
| 4. |
Only I and III |
35. Amongst the following which gas is the least contributor to total global warming?
1. Methane
2. Carbon dioxide
3. Chlorofluorocarbons
4. Nitrous oxide
Botany - Section B
36. Which of the following is the correct description of an anther?
| 1. |
Pollen sac |
| 2. |
The pollen-producing reproductive organ of a flower |
| 3. |
The male gametophyte of angiosperms |
| 4. |
The part of a stamen that contains the pollen |
37. Single-membrane compartment found in plant cells and mainly functioning in the conversion of fat into sugars are:
1. Peroxisomes
2. Glyoxysomes
3. Lysosomes
4. Ribosomes
38. Apoplastic movement of water in plants:
| 1. |
involves crossing the cell membrane multiple times |
| 2. |
is through mass flow |
| 3. |
accounts for a very small fraction of water flow in roots |
| 4. |
is relatively much slower than the symplastic flow |
39. All the following regarding diffusion, as a means of transport, are true except:
| 1. |
Molecules move in a random fashion |
| 2. |
It is not dependent on a living system |
| 3. |
Diffusion of solids rather than diffusion in solids is more likely |
| 4. |
It is the only means of gaseous movement within the plant body |
40. The term ‘archegonium’ for the female sex organ is not used for:
1. Liverworts
2. Pteridophytes
3. Angiosperms
4. Gymnosperms
41. When plants like potato and sugarcane are cultivated, the site of origin of the new plantlets
| 1. |
is invariably the nodes present in the modified stems |
| 2. |
is usually the nodes present in the modified stems but can sometimes be the internodes as well |
| 3. |
is usually the internodes present in the modified stems but can sometimes be the nodes as well |
| 4. |
is invariably the internodes present in the modified stems |
42. What is the direct source of energy for the production of ATP by ATP synthase?
| 1. |
the electron transport chain |
| 2. |
the proton gradient |
| 3. |
substrate-level phosphorylation |
| 4. |
the oxidation reactions occurring during respiration |
43. Antirrhinum majus with pink flowers was cross pollinated by Antirrhinum majus with white flowers. What proportion of progeny plants are expected to have white flowers?
1. 0
2. ¼
3. ½
4. ¾
44. Animal cells cannot be transformed with the help of:
| 1. |
Microinjection |
2. |
Ti Plasmid |
| 3. |
Retroviral vectors |
4. |
Liposomes |
45. In drought-prone areas, sprays of which of the following can be used as anti-transpirant?
| 1. |
ABA |
2. |
GA3 |
| 3. |
IAA |
4. |
Benzyl amino purine
|
46. After the primary producer level in an ecosystem:
| 1. |
less and less amount of new chemical energy is added at successive trophic levels |
| 2. |
no new chemical energy is added at successive trophic levels |
| 3. |
more and more amount of new chemical energy is added at successive trophic levels |
| 4. |
less amount of new chemical energy is added at primary consumer level and then the amount of new chemical energy added to successive levels becomes more and more |
47. The number of molecules of oxygen gas used during the glycolysis of one glucose molecule is:
48. Identify the correct statements:
| I: |
Chipko movement aimed at protecting trees and forests. |
| II: |
Joint Forest Management originated in West Bengal in 1980's in India. |
| III: |
The Amrita Devi Bishnoi Wildlife Protection Award is a national award instituted by the government of India for wildlife conservation. |
| 1. |
Only I and II |
| 2. |
Only I and III |
| 3. |
Only II and III |
| 4. |
I, II and III |
49. In a plant, genes A, B and C are located on different autosomes. Two plants with genotypes AaBbCc are inter-crossed. The number of phenotypes and genotypes that can occur in their progeny will respectively be:
| 1. |
6 and 8 |
2. |
8 and 9 |
| 3. |
4 and 16 |
4. |
8 and 27 |
50. In a cytochrome molecule, which of the following actually accepts and releases electrons?
| 1. |
oxygen |
2. |
zinc |
| 3. |
carbon |
4. |
iron
|
Zoology - Section A
51. A morphological arrangement in which the base of the tooth is completely enclosed in a deep socket of bone is called as:
1. Acrodont
2. Thecodont
3. Pleurodont
4. Heterodont
52. Identify the incorrectly matched pair:
| 1. |
Medulla oblongata |
Gastric secretions |
| 2. |
Hypothalamus |
Control of body temperature |
| 3. |
Limbic system |
Behaviour |
| 4. |
Association areas |
Purely sensory areas in the cerebrum |
53. ELISA works on the principle of:
| 1. |
antigen-antibody interaction |
| 2. |
radioactive probe tagging |
| 3. |
culture of microbe in an artificial medium |
| 4. |
histopathology |
54. It is desirable to use restriction enzymes that create sticky ends in recombinant DNA technology procedures because this:
| 1. |
facilitates the action of DNA ligase |
| 2. |
makes selection of recombinant DNA possible |
| 3. |
increases the copy number of rDNA per cell |
| 4. |
will create multiple fragments of the vector DNA |
55. The lac operon can be described as:
| 1. |
an inducible operon with a positive regulation by the repressor protein |
| 2. |
an inducible operon with a negative regulation by the repressor protein |
| 3. |
a repressible operon with a positive regulation by the repressor protein |
| 4. |
a repressible operon with a negative regulation by the repressor protein |
56. Consider the two statements:
| I: |
Mules are mostly infertile. |
| II: |
Mule is the hybrid of a male donkey and a female horse. |
| 1. |
Both I and II are correct and II explains I |
| 2. |
Both I and Ii are correct but II does not explain I |
| 3. |
I is correct but Ii is incorrect |
| 4. |
Both I and Ii are incorrect |
57. Both Molluscs and Echinoderms:
| 1. |
exhibit radial symmetry as adults |
| 2. |
have segmented bodies |
| 3. |
are diploblastic |
| 4. |
are coelomates |
58. The mode of asexual reproduction shown in the given figure is:

1. Budding
2. Fission
3. Fragmentation
4. Parthenogenesis
59. The hormone, essential for maintaining pregnancy, secreted in large amounts by the corpus luteum is:
| 1. |
Estrogen |
| 2. |
Progesterone |
| 3. |
LH |
| 4. |
FSH |
60. The ‘neck’ in cockroach is:
1. an extension of the head capsule
2. the proximal tergite
3. the proximal sternite
4. an extension of prothorax
61. Which of the following values for the given parameter is incorrect?
| 1. |
Partial pressure of oxygen in alveolar air |
104 mm Hg |
| 2. |
Partial pressure of carbon dioxide in deoxygenated blood |
45 mm Hg |
| 3. |
Transport of carbon dioxide in blood as bicarbonate |
70 % |
| 4. |
Transport of carbon dioxide as carbaminohemoglobin |
7 % |
62. Oral contraceptive combination pills:
| I: |
Inhibit ovulation |
| II: |
May prevent implantation |
| III: |
Retard passage of sperms through the cervix |
| 1. |
Only I and II are correct |
| 2. |
Only I and III are correct |
| 3. |
Only II and III are correct |
| 4. |
I, II and III are correct |
63. Consider the two given statements:
| I: |
The life cycles of endoparasites are more complex than that of the ectoparasites. |
| II: |
The morphological and anatomical features of endoparasites are greatly simplified. |
| 1. |
Both I and II are correct and II explains I |
| 2. |
Both I and II are correct but II does not explain I |
| 3. |
I is correct but II is incorrect |
| 4. |
Both I and II are incorrect |
64. Consider the following statements:
| I: |
Glomerulus is a tuft of capillaries formed by the efferent arteriole |
| II: |
Afferent arteriole forms the peritubular capillary network |
| III: |
Vasa recta is absent or highly reduced in juxtamedullary nephrons |
| 1. |
Only III is correct |
| 2. |
I is correct but II and III are incorrect |
| 3. |
I, II and III are incorrect |
| 4. |
I and II are correct |
65. Given below are two statements:
| Assertion (A): |
Most cancers are treated by a combination of surgery, radiotherapy, and chemotherapy |
| Reason (R): |
Combination therapy is curative for all types of cancers |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
66. In a plasmid such as pBR322:
| I: |
Ori controls the copy number of DNA per cell |
| II: |
Multiple cloning sites are recognition sequences of common restriction enzymes |
| III: |
Genes for antibiotic resistance are used as selectable markers |
| 1. |
Only I and II are correct |
| 2. |
Only I and III are correct |
| 3. |
Only II and III are correct |
| 4. |
I, II, and III are correct |
67. Plasmids are good cloning vectors mainly because:
| 1. |
they are circular |
| 2. |
they replicate autonomously |
| 3. |
they are extra-chromosomal DNA molecules found in many bacterial cells |
| 4. |
they carry genes vital for normal survival and reproduction |
68. Match each item in column I with one in column II and select the correct match from the codes given:
|
Column I |
|
Column II |
| A |
Hibernation |
P |
Bears in winter months |
| B |
Aestivation |
Q |
Air-breathing land snails |
| C |
Diapause |
R |
Zooplankton in lakes and ponds |
Codes:
|
A |
B |
C |
| 1. |
P |
Q |
R |
| 2. |
Q |
P |
R |
| 3. |
P |
R |
Q |
| 4. |
R |
Q |
P |
69. The thick filaments in the ‘A’ band are also held together in the middle of this band by a thin fibrous membrane called:
| 1. |
I-band |
2. |
Z-line |
| 3. |
M-line |
4. |
Myofibril |
70. All the following statements regarding Medical Termination of Pregnancy are correct except:
| 1. |
It accounts for about 1/5th of the total number of pregnancies conceived in a year |
| 2. |
It was legalized by the Government of India in 1975 |
| 3. |
It is considered safe during the first trimester of pregnancy |
| 4. |
It is legalized induced abortion |
71. Identify the incorrect statement regarding HIV/AIDS:
| 1. |
HIV is a retrovirus |
| 2. |
HIV can be transmitted from an infected mother to her child through placenta. |
| 3. |
The time lag between infection and the appearance of AIDS symptoms is 7-10 days. |
| 4. |
HIV destroys helper T lymphocytes. |
72. Which of the following will not be an example of passive immunity?
| 1. |
Foetus receiving antibodies from their mother, through placenta. |
| 2. |
Immunity conferred by IgA antibodies present in colostrums. |
| 3. |
Giving antivenom [containing antibodies against venom] after snake bite |
| 4. |
A person developing immunity after natural infection by a pathogen |
73. Match each item in Column I with the one in Column II and select the correct match from the codes given:
|
Column I |
|
Column II |
| A |
Pivot joint |
P |
Between carpal and metacarpal of thumb |
| B. |
Saddle joint |
Q |
Between atlas and axis vertebrae |
| C |
Gliding joint |
R |
Between carpal bones |
| D |
Hinge joint |
S |
Humero-ulnar joint |
Codes:
|
A |
B |
C |
D |
| 1. |
P |
Q |
R |
S |
| 2. |
Q |
P |
S |
R |
| 3. |
Q |
P |
R |
S |
| 4. |
P |
Q |
S |
R |
74. During post-transcriptional modification of hnRNA in eukaryotes:
| I: |
Capping is done at the 5’ end and tailing at the 3’ end |
| II: |
Exons are removed from the split gene to form the cistron |
| 1. |
Only I is correct |
| 2. |
Only II is correct |
| 3. |
Both I and II are correct |
| 4. |
Both I and II are incorrect |
75. The untranslated regions:
| I: |
are present at only the 5’ end of the mature mRNA in eukaryotes |
| II: |
are not required during translation and hence are spliced out of the mature mRNA |
| 1. |
Only I is correct |
| 2. |
Only II is correct |
| 3. |
Both I and II are correct |
| 4. |
Both I and II are incorrect |
76. Dominance:
| 1. |
is an autonomous feature of an allele |
| 2. |
is inherent in the phenotype expressed by an allele |
| 3. |
is a relationship between two alleles of a gene and their associated phenotypes |
| 4. |
determines whether an allele is deleterious, neutral or advantageous |
77. Which of the following organelles in a eukaryotic cell have circular DNA, 70 S ribosomes and make some of their own proteins?
| 1. |
Golgi apparatus and Vacuole |
| 2. |
Endoplasmic reticulum and Golgi apparatus |
| 3. |
Plastids and mitochondria |
| 4. |
Nucleolus and ribosomes |
78. Monkey, gorilla, gibbon, tiger, cat and dog do not belong to the same:
| 1. |
Phylum |
2. |
Order |
| 3. |
Class |
4. |
Kingdom |
79. Which of the following is not true regarding phenylketonuria?
| 1. |
It is inherited as an autosomal recessive condition. |
| 2. |
The affected individual lacks the enzyme phenylalanine hydroxylase. |
| 3. |
The affected individuals lack the capability of converting tyrosine to phenylalanine. |
| 4. |
Clinical features include mental retardation and a reduction in hair and skin pigmentation. |
80. Which diseases given in
Column-I is not correctly matched with sign and symptoms in
Column-II?
|
Column-I |
Column-II |
| 1. |
Filariasis |
Inflammation and swelling in lower limbs and scrotum |
| 2. |
Ringworm |
Internal bleeding, muscular pain, fever, and anaemia |
| 3. |
Amoebiasis |
Abdominal pain, stools with excess mucous, and blood clots |
| 4. |
Pneumonia |
Fever, chills, cough, breathing difficulties |
81. Alec Jeffreys pioneered the technique for:
1. DNA profiling
2. Amplification of DNA
3. Separation of DNA fragments in gel electrophoresis
4. Artificial synthesis of gene
82. The tunica media in veins when compared to the tunic media in arteries:
1. is much thinner
2. is much thicker
3. is equally developed
4. is absent
83. Identify the incorrect statement:
| 1. |
Arrangement of axonemal microtubules in cilia and flagella is described as 9 + 2 |
| 2. |
Arrangement of microtubules in centriole is described as 9 + 0 |
| 3. |
Centrosome is involved in spindle formation in plant cell division |
| 4. |
Centrioles form the basal body of cilia and flagella |
84. All the following can be performed by LAB except:
| 1. |
Coagulation and partial digestion of milk proteins |
| 2. |
Increasing vitamin B12 in curd |
| 3. |
Checking disease- causing microbes in our stomach |
| 4. |
Producing large amount of carbon dioxide in fermentation of Swiss cheese |
85. In the human genome:
1. there are 3164.7 billion base pairs
2. largest known gene is HbS
3. more than 20 percent part codes for proteins
4. chromosome 1 has most genes
Zoology - Section B
86. The testes descend into the scrotum during foetal life. Undescended testes are associated with reduced fertility. Which of the following, if true, will explain normal fertility in descended testes?
| 1. |
About 80% of undescended testes descend by the first year of life. |
| 2. |
Most such cases are actually anorchia – an absence of testes. |
| 3. |
The temperature of the testes in the scrotum is at least a few degrees cooler than in the abdomen. |
| 4. |
There is a high rate of anomalies of the epididymis in boys with undescended testes. |
87. The hypothalamus and the pituitary gland mainly control the menstrual cycle. A series of physiological and anatomical processes of puberty culminates in menarche. Which of the following is the most important causal reason for the onset of puberty?
| 1. |
The release of pulses of GnRH by the hypothalamus |
| 2. |
The release of gonadotropins by the anterior pituitary |
| 3. |
Inhibition of GnRH due to increase in circulating gonadotropins |
| 4. |
Secretion of estrogen by the ovaries in response to pituitary hormones |
88. Dictyotene is:
| 1. |
Abnormal meiosis in fungi |
| 2. |
Prolonged resting phase in oogenesis |
| 3. |
Failure of homologues to separate during meiosis |
| 4. |
State of hypermotility in sperms |
89.
The '?' in the given figure shows:

| 1. |
Tight junctions |
2. |
Adhering junctions |
| 3. |
Gap junctions |
4. |
Desmosomes |
90. The spine of the scapula bone is located on its:
| 1. |
lateral border |
2. |
medial border |
| 3. |
ventral surface |
4. |
dorsal surface |
91. The karyotype of a healthy human being will not show:
| 1. |
Metacentric chromosomes |
| 2. |
Submetacentric chromosomes |
| 3. |
Acrocentric chromosomes |
| 4. |
Telocentric chromosomes |
92. In males, the Follicle Stimulating Hormone:
| I: |
stimulates primary spermatocytes to undergo the first division of meiosis, to form secondary spermatocytes. |
| II: |
enhances the production of androgen-binding protein by the Sertoli cells of the testes |
| 1. |
Only I |
2. |
Only II |
| 3. |
Both I and II |
4. |
Neither I nor II |
93. Identify the correct statement:
1. Enterokinase activates all pancreatic proteolytic enzymes
2. Proenzymes are secreted only by the pancreas
3. Bile activates lipases secreted by the liver
4. Disaccharidases are brush border enzymes
94. An increase in blood flow to the atria of the heart can cause the release of ANF. ANF:
| I: |
is a potent vasoconstrictor |
| II: |
can cause a decrease in blood pressure |
| III: |
acts as a check on RAAS |
1. Only I and II are correct
2. Only III is correct
3. Only II and III are correct
4. Only I is correct
95. Match each item in Column-I with one in Column-II and select the correct match from the codes given:
|
Column-I |
|
Column-II |
| A. |
Heart |
P. |
Synthesis of angiotensinogen |
| B. |
Lungs |
Q. |
Secretion of atrial natriuretic peptide |
| C. |
Kidneys |
R. |
Primary site for conversion of angiotensin I to angiotensin II |
| D. |
Liver |
S. |
Secretion of renin |
Codes:
|
A |
B |
C |
D |
| 1. |
P |
Q |
R |
S |
| 2. |
R |
Q |
P |
S |
| 3. |
Q |
R |
S |
P |
| 4. |
Q |
R |
P |
S |
96. Identify the correct statement:
| 1. |
Unmyelinated neurons in PNS are not surrounded by Schwann cell |
| 2. |
Electrical synapses, being faster, are more common in the human body |
| 3. |
Pia mater is in contact with the brain tissue |
| 4. |
The entire hindbrain and the midbrain constitute the brain stem |
97. In metamerism, there is serial repetition of unit subdivisions of:
| I. |
Ectoderm products |
| II. |
Mesoderm products |
| III. |
Endoderm products |
| 1. |
Only II |
2. |
Only I and II |
| 3. |
Only II and III |
4. |
I, II and III |
98. A double stranded DNA molecule from a bacteriophage is found to consist of 23% adenine bases. The expected percentage of guanine in this DNA molecule will be:
| 1. |
23 % |
2. |
27 % |
| 3. |
46 % |
4. |
54 % |
99. If the heart rate is 60 per minute, the duration of each cardiac cycle will be:
| 1. |
0.8 seconds |
2. |
0.75 seconds |
| 3. |
1.0 second |
4. |
1.2 second |
100. Identify the correctly matched pair:
|
Part of the pituitary |
Hormone synthesized |
| I: |
Pars distalis |
Gonodotropin releasing hormone |
| II: |
Pars intermedia |
Melanocyte stimulating hormone |
| III: |
Pars nervosa |
ADH and oxytocin |
| 1. |
Only III |
2. |
Only II |
| 3. |
Only I and II |
4. |
Only II and III |
Chemistry - Section A
101. In the structure of diborane (B2H6) , the hybridised state of B & the maximum number of atoms which are present in one plane, respectively, are:
| 1. |
sp2 & 4 |
2. |
sp3 & 4 |
| 3. |
sp2 & 6 |
4. |
sp3 & 6 |
102. Which of the following solutions has the highest freezing point?
1. 0.02 M NaCl
2. 0.05 M Urea
3. 0.01 M MgCl2
4. 0.01 M KCl
104. In which of the following complexes, the central atom has sp3 hybridisation?
1. [PtCl4]2-
2. [AuCl4]-
3. [Cu(NH3)4]2+
4. None of the above
105. Van Arkel method of purification of metals involves converting the metal to a
1. Non-volatile stable compound
2. Volatile stable compound
3. Non-volatile unstable compound
4. Volatile unstable compound
106. 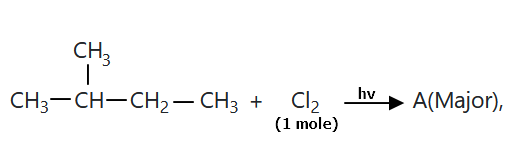
The compound 'A' is:
107. In the esterification reaction, H+ ion attacks on:
1. Oxygen atom of alcohol
2. Doubly bonded oxygen atom of carboxylic acid
3. Singly bonded oxygen atom of carboxylic acid
4. Any of the oxygen atoms
108. Given below are two statements:
| Assertion (A): |
To detect presence of halogens in an organic compound,
some dilute nitric acid is added. |
| Reason (R): |
Dilute HNO3 destroys Na2S and NaCN by oxidation. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
109. Fe(OH)3 is a positive sol. Which electrolyte has the minimum flocculation value for the precipitation of Fe(OH)3 sol?
1. Na3PO4
2. K4[Fe(CN)6]
3. MgSO4
4. NaCl
110. The pH of 10-6 M CH3COOH will be:
(Given: ka of CH3COOH = 1.8 \(\times\) 10-5 & log 4.24 =0.63)
1. 5.37
2. 7.0
3. Slightly more than 6
4. 6.95
111. Which is not the product of hydrolysis of XeF6 :
1. XeO3
2. XeO2F2
3. XeOF4
4. XeOF2
112. Consider the following:
Cl2 | Cl- || Cl- | Cl2
(1atm) (C1) (C2) (1 atm)
For spontaneous cell, which of the following condition, is correct?
1. C1> C2
2. C1 <C2
3. C1 = C2
4. All of the above
113. 
Which \(\pi\) bond is most reactive towards the attack of \(H^+\)?
1. A
2. B
3. C
4. All \(\pi\) bonds have same reactivity
114. Which artificial sweetener has the highest sweetness value in comparison to cane sugar?
1. Aspartame
2. Saccharin
3. Sucralose
4. Alitame
115. The slag formed in the metallurgy of copper is
1. \(CuSiO_3\)
2. \(CaSiO_3\)
3. \(FeSiO_3\)
4. \(MgSiO_3\)
116. Determine the oxidation number of sulfur in Caro's acid (H₂SO₅).
117. For the given reaction:
H2NCOONH4 (s) \( \rightleftharpoons\) 2NH3 (g) + CO2 (g),
Total pressure at equilibrium is found to be 18 atmospheres. The value of Kp for the above equilibrium will be:
| 1. |
72 atm3 |
2. |
144 atm3 |
| 3. |
432 atm3 |
4. |
864 atm3 |
118. Formal charges of the central atom in \(SO_3\) , \(NO^-_3\) and \(NH^+_4\) are, respectively:
1. 0 , +1 , +1
2. +2 , +1 , +1
3. +2 , -1 , +1
4. 0, -1 , +1
119. 2.5 litre mixture of CO and CO2 is passed through red hot charcoal in a tube. The new volume becomes 3.5 litre. All measurements are made at same temperature and pressure. Percentage composition of original mixture by volume will be:
1. CO = 50%, CO2 = 50%
2. CO = 60%, CO2 = 40%
3.CO = 40%, CO2 = 60%
4.CO = 20%, CO2 = 80%
120. When hard water is reacted with Calgon then Calcium or Magnesium of hard water are removed as
1. Cationic form
2. Anionic form
3. Can be cationic or anionic form
4. Salt
121. Case Study:
A buffer solution is defined as a solution whose pH remains practically constant even when small amounts of an acid or a base are added to it.
Types of buffer solutions :
| (i) |
Acidic buffer: It is a solution of a mixture of a weak acid and a salt of this weak acid with a strong base (e.g. CH3COOH + CH3COONa) |
| (ii) |
Basic buffer: It is the solution of a mixture of a weak base and a salt of this weak base with a strong acid (e.g. NH4OH + NH4Cl) |
Henderson's equation is used to determine pH of buffer mixtures of differ types:
for acidic buffer Henderson's equation is
pH= pKa + log \([Salt] \over [Acid]\) (ka = ionisation constant of weak acid )
for basic buffer Henderson's equation is :
POH = Pkb + log \([Salt] \over [Base]\) (kb = ionisation constant of weak base )
When CH3COONa is added to CH3COOH solution, the pH of the solution is:
1. Decreases
2. Increases
3. Remains unchanged
4. First decreases and then increases
122. Insulin is a type of protein. How many amino acids makeup insulin?
123. Which, of the following, is not a ferromagnetic substance?
1. CrO2
2. Fe
3. Fe3O4
4. Ni
124. The correct order of electron affinity is given in:
1. O > S > Se
2. S > O > Se
3. Se > O >S
4. S > Se > O
125. Which, of the following, electrolytes has the maximum flocculation value for the precipitation of \(Fe(OH)_3\) sol?
1. \(Na_3PO_4\)
2. \(K_4 [Fe(CN)_6]\)
3. \(BaSO_4\)
4. \(NaCl\)
126. The ionization energy of the hydrogen atom is 13.6 eV and the first ionization energy of the sodium atom is 5.1 eV. The effective nuclear charge experienced by the valence electron of the sodium atom is:
(Round off to one decimal place)
1. 2.8
2. 1.8
3. 1.2
4. 2.2
127. Case Study Question:
The relative location of the bond pair and the lone pair is decided by Bent's rule. The rule states that the more electronegative atom prefers to stay in the orbital having less s character while the lone pair prefers to stay in the orbital having more s character. In molecules with trigonal bipyramidal (TBP) geometry, the more electronegative atom prefers the axial position while the lone pair prefers the equatorial position.
Which molecule, among the following, has non-zero dipole moment?
1. PCl3F2
2. PCl3Br2
3. PCl3(CH3)2
4. Both (2) and (3)
128. In aqueous medium, K4[Fe(CN)6] is 40% dissociated. The value of van't Hoff factor (i) for K4[Fe(CN)6] will be:
129. Assertion (A): PAN (Peroxy Acetyl Nitrate) is a secondary pollutant.
Reason (R): PAN is formed by the interaction of \(O_3\) and \(NO_2.\)
1. Both (A) and (R) are true and (R) is the correct explanation of (A).
2. Both (A) and (R) are true but (R) is not the correct explanation of (A).
3. (A) is true but (R) is false.
4. (A) and (R) both are false.
130. \(\pi\) bond of which of the following bond is strongest?
1. Double bond
2. Triple bond
3. Same strength in double as well as triple bond
4. Unpredictable & cannot be said
131. Which element has the highest melting point?
1. Cr
2. Fe
3. Mo
4. Cu
132. In Solvay process (for the manufacture of Na2CO3), the compound added for the recovery of ammonia and the bi-product formed, respectively, are
1. Ca(OH)2 & CaCl2
2. NaOH & NH4Cl
3. NaCl & NH4Cl
4. NaOH & CaCl2
133. What is the correct order of reactivity for the following compounds in an \(S_N 1\) reaction?

| 1. |
II > I > III |
2. |
III > II > I |
| 3. |
I > II > III |
4. |
I > III > II |
134. Which of the following oxides is the most basic in nature?
1. Bi2O3
2. Bi2O5
3. As2O3
4. Sb2O5
135. At equilibrium, which of the following is always zero?
| 1. |
\(\Delta G_{system}\) |
2. |
\(\Delta S_{Total}\) |
| 3. |
\(\Delta S_{system}\) |
4. |
Both (1) and (2) |
Chemistry - Section B
136. In the Ellingham diagram, the graph between
\(\Delta G\) vs T has a negative slope for which of the following conversion
1.
\(C \rightarrow CO_2\)
2.
\(Mg \rightarrow MgO\)
3.
\(C \rightarrow CO\)
4.
\(Al \rightarrow Al_2O_3\)
137. Calculate the approximate mass of
\(CO_2\) produced when 1 gm of butane
\((C_4H_{10})\) is burned with
an excess of
\(O_2\) to form
\(CO_2\).
| 1. |
1 g |
2. |
2 g |
| 3. |
3 g |
4. |
4 g |
138. For the reaction,
\(H_2NCOONH_4 (s) \rightleftharpoons2 NH_3(g) + CO_2(g)\), the total pressure at equilibrium is 15 atmospheres. It can be concluded that the value of \(K_P\) will be:
1. 15 atm3
2. 50 atm3
3. 500 atm3
4. 100 atm3
139. How many moles of KMnO4 are required to oxidize one mole of KI in the alkaline medium?
1. 1/2
2. 1
3. 2
4. 2/3
140. From the given set of 14th group elements, the element having the lowest ionisation potential is:
141. The rate of diffusion is highest for:
1. \(CH_4\)
2. \(SO_2\)
3. \(NO_2\)
4. \(NH_3\)
142. In Heisenberg's uncertainty principle, if uncertainty in the position
\((\Delta x)\) is equal to the uncertainty in the momentum
\((\Delta p) \) then uncertainty in the velocity
\((\Delta v) \) will be:
| 1. |
\(\dfrac{1}{m}\sqrt{\dfrac{h}{2\pi}}\) |
2. |
\(\dfrac{1}{2m}\sqrt{\dfrac{h}{\pi}}\) |
| 3. |
\(\dfrac{1}{m}\sqrt{\dfrac{h}{\pi}}\) |
4. |
\(\dfrac{1}{2m}\sqrt{\dfrac{h}{2\pi}}\) |
143. 3% (W/V) solution of glucose is isotonic with 1% (W/V) solution of a non-volatile and non-electrolyte substance. The molecular mass of the substance would be:
1. 180 g/mol
2. 360 g/mol
3. 420 g/mol
4. 60 g/mol
144. How many functional isomers and how many pairs of functional isomers are there for the molecular formula \(C_4H_{10}O\)?
1. 2 & 2
2. 2 & 4
3. 2 & 10
4. 2 & 12
145. Which of the following compounds has the highest lattice energy?
1. LiCl
2. BeO
3. LiF
4. MgO
146. When sodium chloride crystal is heated in sodium metal vapors then it gives the appearance of yellow color. It is due to
1. F - center defect
2. Anion deficiency defect
3. Metal excess defect
4. All of the above
147. When one mole ethyl methyl ketone is oxidised with acidic K2Cr2O7 then the product formed is:
1. Two moles of CH3COOH
2. One mole of C2H5COOH and one mole of HCOOH
3. One mole of C2H5COOH and one mole of CO2
4. One mole of CH3COOH and 2 mole of HCOOH
148. The IUPAC name of [Pt(NH
3)
2Cl
2] [PtBr
4] is:
| 1. |
Diamminedichloridoplatium (II) tetrabromidoplatinate (IV) |
| 2. |
Diamminedichloridoplatinum (III) tetrabromidoplatinate (III) |
| 3. |
Diamminedichloridoplatinum (IV) tetrabromidoplatinate (II) |
| 4. |
Diamminedichloridoplatinum (IV) tetrabromidoplatinum (II) |
149. When \(CuSO_4\) solution using copper electrodes is electrolysed, pH of the solution
1. Increases
2. Decreases
3. Remains the same
4. Firstly increases and then decreases
150. Consider the following reaction:
\(A(g) \rightarrow B(g) + C(g) + D(g)\)
If the initial pressure is \(P^o\) and pressure at 't' time is \(P_t (P_t >P^o)\). If the above reaction is of first order, the rate expression will be
1. \(K = {2.303 \over t}log {2p^o \over 2p^o-P_t}\)
2. \(K = {2.303 \over t}log {p^o \over P_t}\)
3. \(K = {2.303 \over t}log {2p^o \over 3p^o-P_t}\)
4. \(K = {2.303 \over t}log {p^o \over 3p^o-P_t}\)
Physics - Section A
151. \(D_1,D_2\) are ideal diodes and the output
\((V_{AB})\) of the transformer is
\(10\) V (RMS). The current through the resistor is:
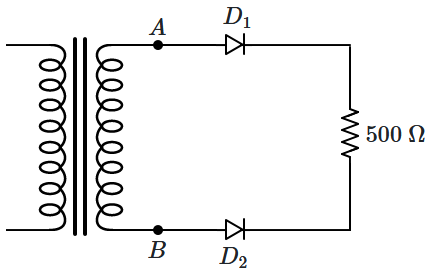
1. zero
2.
\(20\) mA
3.
\(40\) mA
4.
\(20\sqrt2\) mA
152. Photons and electrons of the same wavelength are compared. Which one carries larger momentum?
| 1. |
photon |
| 2. |
electron |
| 3. |
neither, since both have equal momenta |
| 4. |
it could be either, depending on the energy |
153. An ideal monoatomic gas at a temperature of
\(300\) K and a pressure of
\(10\) atm is suddenly allowed to expand into vacuum so that its volume is doubled. No exchange of heat is allowed to take place between the gas and its surroundings during the process. After equilibrium is reached, the final temperature is:
| 1. |
\(300\) K |
2. |
\(\dfrac{300}{2^{5/3}}\) K |
| 3. |
\(\dfrac{300}{2^{2/3}}\) K |
4. |
\(600\) K |
154. A capacitor is constructed by taking metallic circular discs of radius
\(r\) placed face-to-face with a separation of
\(d.\) A dielectric slab is inserted into the space between the plates so that it fills the entire width, but only half the area between the plates. The dielectric constant is
\(K.\) Then, the capacitance is:
| 1. |
\(\dfrac{K\varepsilon_0\pi r^2}{d}\) |
2. |
\(\dfrac{K\varepsilon_0\pi r^2}{2d}\) |
| 3. |
\(\dfrac{K+1}{2}\dfrac{\varepsilon_0\pi r^2}{d}\) |
4. |
None of the above |
155. Plane waves of light of wavelength
\(\lambda\) are incident onto a convex lens, and the beam is brought to a focus. A plane slab of thickness
\(t\) having refractive indices
\(\mu_1,~\mu_2\) in the upper and lower halves is placed parallel to the incoming wavefronts. The phase difference between the wavefronts at the focus, coming from the upper and lower halves of the slab is:

| 1. |
\(\dfrac{2 \pi}{\lambda}\left[\left(\mu_{1}-1\right) t+\left(\mu_{2}-1\right) t\right]\) |
| 2. |
\(\dfrac{2 \pi}{\lambda}\left(\mu_{1}-\mu_{2}\right) t\) |
| 3. |
\(\dfrac{2 \pi}{\lambda}\left(\dfrac{t}{\mu_{1}}-\dfrac{t}{\mu_{2}}\right)\) |
| 4. |
\(\dfrac{2 \pi}{\lambda}\left(\dfrac{t}{\mu_{1}}+\dfrac{t}{\mu_{2}}\right)\) |
156. An inductor
\((L)\) and a resistor
\((R)\) are connected in series and a battery is connected, as shown in the figure. Once the current becomes steady, the power in the resistance is
\(P_R\) and the energy stored in the inductor is
\(U_L.\) The switch is suddenly (and smoothly) toggled to the position
\(B\) allowing the inductor to discharge. The time in which the energy stored becomes
\(\dfrac12\) its initial value is:

| 1. |
\(\dfrac{U_L}{P_R}\) |
2. |
\(\dfrac{U_L~\mathrm {ln}2}{P_R}\) |
| 3. |
\(\dfrac{2U_L~\mathrm{ln 2}}{P_R}\) |
4. |
\(\dfrac{2U_L}{P_R}\) |
157. A light rod
\(AB\) is hinged at
\(A\) so that it is free to rotate about
\(A.\) It is initially horizontal with a small block of mass
\(m\) attached at
\(B,\) and a spring
(constant - \(k\)) holding it vertically up at its mid-point. The time period of vertical oscillations of the system is:

| 1. |
\(2 \pi \sqrt{\dfrac{m}{k}} \) |
2. |
\(\pi \sqrt{\dfrac{m}{k}} \) |
| 3. |
\(4\pi \sqrt{\dfrac{m}{k}}\) |
4. |
\(\dfrac{\pi}{2} \sqrt{\dfrac{m}{k}}\) |
158. The resistance between
\(A,B\) is found to be
\(500~\Omega\) while that between
\(A,C\) is
\(400~\Omega.\) The minimum possible value of
\(R_2\) is:

1.
\(100~\Omega\)
2.
\(200~\Omega\)
3.
\(400~\Omega\)
4.
\(900~\Omega\)
159. Given below are two statements:
| Statement I: |
Radioactivity is a nuclear reaction, where the nucleus disintegrates spontaneously and particles are emitted. |
| Statement II: |
During the process of radioactive decay, the parent and daughter nuclei have the same number of protons. |
| 1. |
Statement I is incorrect and Statement II is correct. |
| 2. |
Both Statement I and Statement II are correct. |
| 3. |
Both Statement I and Statement II are incorrect. |
| 4. |
Statement I is correct and Statement II is incorrect. |
160. A block of wood is immersed in water (assume that the wood is impervious to water), and it floats half immersed. It is placed in an elevator which is accelerating upward. Then,
| 1. |
the block floats, slightly more of it out of the water. |
| 2. |
the block floats, slightly less of it out of the water. |
| 3. |
the block floats, half-immersed. |
| 4. |
the block sinks completely. |
161. A household ac circuit has an applied voltage of
\(220\) V (RMS) and the current flowing through the circuit is
\(2.2\) A (RMS), the phase difference between them being
\(60^\circ.\) Then:
| 1. |
the impedance in the circuit is \(100~\Omega.\) |
| 2. |
the resistance in the circuit is \(200~\Omega.\) |
| 3. |
the power dissipated is \(484\) W. |
| 4. |
all the above are true. |
162. A block of \(1\) kg is released from the top of a smooth curve \(\mathrm{AB},\) and then it encounters a rough surface \(\mathrm{BC},\) coming to rest at \(\mathrm{C}.\) The work done by friction is:
(take \(g=10\) m/s2)

| 1. |
\(25\) J |
2. |
\(50\) J |
| 3. |
\(-25\) J |
4. |
\(-50\) J |
163. A block of mass \(m,\) placed on a rough incline (as shown) – is observed to remain at rest. The coefficient of friction is \(\mu.\) The net force exerted by the incline on the block equals: (in magnitude)

1. \(mg \cos\theta +\mu mg\cos\theta\)
2. \(mg\cos\theta\sqrt{1+\mu^2}\)
3. \(mg\sin\theta\)
4. \(mg\)
164. A magnetic needle suspended in the earth's magnetic field shows a dip of \(45^{\circ}.\) The horizontal component of the earth's magnetic field is \(B_H.\) The magnetic field of the earth equals
1. \(B_H\)
2. \(\frac{B_H}{\sqrt2}\)
3. \(B_H\sqrt2\)
4. \(2B_H\)
165. A particle moves around a circle with a unique uniform speed in each revolution. After the first revolution and during the \(2\)nd revolution: its speed doubles; and during the \(3\)rd revolution, its speed becomes \(3\) times the initial speed and so on. The time for the \(1\)st revolution is \(12\) sec. The average time per revolution, for the first four revolutions, is:
1. \(4.8\) s
2. \(9.6\) s
3. \(6.25\) s
4. \(6\) s
166. The radiation emerging from a furnace (blackbody) is found to have a most probable wavelength
\(\lambda_m\) and the gas molecules (air) emerging from it have an RMS speed
\(v.\) As the temperature of the furnace is varied:

| 1. |
\(\lambda_m\propto v \) |
2. |
\(\lambda_m\propto \dfrac1v \) |
| 3. |
\(\lambda_m\propto v^2 \) |
4. |
\(\lambda_m\propto \dfrac1{v^2} \) |
167. A heavy uniform rope
\(PQ\) is suspended from the ceiling. The lowest end of the rope is given a sharp transverse "shake" (or vibration) so as to cause a pulse. This pulse travels upward. As it travels upward, its speed:
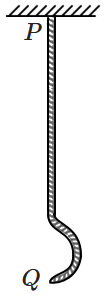
| 1. |
increases |
2. |
decreases |
| 3. |
first increases and then decreases |
4. |
remains constant |
168. Which, of the following, is dimensionless?
| 1. |
\(\text{impedance}\times\text{conductance} \) |
2. |
\(\dfrac{\text{emissive power}}{\text{emissivity}}\) |
| 3. |
\(\dfrac{\text{electric field}}{\text{magnetic field}}\) |
4. |
\(\dfrac{\text{inductance}}{\text{capacitance}}\) |
169. The output of the above circuit is:

| 1. |
(\(A\) AND \(B\)) |
2. |
(\(A\) OR \(B\)) |
| 3. |
(\(B\) OR \(C\)) |
4. |
none of the above |
170. A ray of light is incident on a mirror which is lying on the \(x\text-y\) plane. The reflected ray travels along the direction of the vector \(3\hat i-4\hat j+2\hat k. \) The incident ray must be along:
1. \(-3\hat i+4\hat j+2\hat k\)
2. \(3\hat i-4\hat j-2\hat k\)
3. \(-3\hat i-4\hat j+2\hat k\)
4. \(-3\hat i+4\hat j-2\hat k\)
171. At the moment of projection of a projectile (mass: \(m\), initial speed: \(u\), angle of projection: \(\theta\)) the power due to the force of gravity is:
1. \(-mg u\)
2. \(mg u \cos\theta\)
3. \(-mgu \cos^2\theta\)
4. \(-mg u \sin\theta\)
172. A projectile is fired so as to give a maximum horizontal range of
\(1\) km. What would be the maximum height reached by it if it were to be fired vertically upward?
| 1. |
\(2\) km |
2. |
\(1\) km |
| 3. |
\(\dfrac12\) km |
4. |
\(\dfrac14\) km |
173. An n-p-n transistor is connected in a common-emitter configuration and it is "ON". If VE, VB, VC are the average (dc) voltages at the emitter, base, and collector, then:
1. VE > VB > VC
2. VE < VB < VC
3. VB > VE > VC
4. VB < VE < VC
174. The centre-of-mass of a combination of a hemispherical shell and a cylindrical shell, both having the same height and radii and same mass, lies at a distance \(h\) from the centre of the hemisphere. Then, \(h\) equals:

| 1. |
\(\dfrac{R}{2}\) |
2. |
\(\dfrac{R}{\pi}\) |
| 3. |
\(\dfrac{2R}{\pi}\) |
4. |
zero |
175. The electric field, at the centre of a square with charges placed at its four vertices as shown in the figure, is:
\(\left(k=\dfrac{1}{4\pi\varepsilon_0}\right)\)

| 1. |
zero |
2. |
\(4\dfrac{kq}{a^2}\) |
| 3. |
\(2\dfrac{kq}{a^2}\) |
4. |
\(2\sqrt2\dfrac{kq}{a^2}\) |
176. Two identical capacitors, each of capacitance
\(C\), are connected in series and are charged by means of an ideal battery of emf
\(E\). They are disconnected and reconnected in parallel and connected to the same battery. During this reconnection, the positive terminals of the capacitors are connected to the positive terminal of the battery and their negative terminals are similarly connected together. Let, the work done by the battery during the first connection be
\(W_1\), and during the second be
\(W_2\). Then,
| 1. |
\(W_1=W_2\) |
2. |
\(2W_1 =W_2\) |
| 3. |
\(W_1 = 2W_2\) |
4. |
\(4W_1 = W_2\) |
177. The moment of inertia of a uniform solid sphere of mass \(m\) and radius \(R\) about a diameter equals the moment of inertia of a solid uniform cylinder of radius \(r\) and height \(2r,\) about its axis. Their masses are the same. Then:
1. \(3r^{2}=4R^{2}\)
2. \(3r^{2}=2R^{2}\)
3. \(5r^{2}=2R^{2}\)
4. \(5r^{2}=4R^{2}\)
178. A large cylindrical piece of a dense solid elastic metal stands on its end as shown in the figure. The metal is uniform and isotropic. The stress in the material as a function of height is shown correctly by:

179. An equimolar mixture of
helium \(\mathrm{(He)}\) and
hydrogen \(\mathrm{(H_2)}\) gases is kept in a vessel at a temperature of
\(500~\text{K}.\) Then:
| 1. |
helium and hydrogen molecules have the same kinetic energy on average. |
| 2. |
RMS speeds of helium and hydrogen molecules are equal. |
| 3. |
the translational kinetic energy of hydrogen and helium molecules is equal. |
| 4. |
all of the above are true. |
180. The acceleration due to gravity, \(g\), near a spherically symmetric planet's surface decreases with height, \(h\) according to the relation:
\(g(h)= g_s-k\cdot h\), where \(h\ll\) the radius of the planet.
The escape speed from the planet's surface is:
| 1. |
\(\dfrac{g_s}{2\sqrt k}\) |
2. |
\(\dfrac{g_s}{\sqrt k}\) |
| 3. |
\(\dfrac{2g_s}{\sqrt k}\) |
4. |
\(g_s\sqrt{\dfrac{2}{k}} \) |
181. Identical cells are connected to identical square wire loops as shown in the two diagrams, and the magnetic fields are respectively
\(B_1\) and
\(B_2\) at the centres.

Then, we can conclude that:
1.
\(B_1>0, B_2=0 \)
2.
\(B_1> B_2>0 \)
3.
\(B_2> B_1>0 \)
4.
\(B_1=0, B_2=0 \)
182. Photons of wavelength
\(\lambda\) cause the emission of photoelectrons from a metallic surface, the de-Broglie wavelength of the fastest photoelectron being
\(\lambda_d\). A graph of
\(\dfrac{1}{\lambda} \text { vs } \dfrac{1}{\lambda_{d}}\) is:
| 1. |
a straight line passing through the origin. |
| 2. |
a circle. |
| 3. |
an ellipse. |
| 4. |
a parabola. |
183. Given below are two statements:
| Statement I: |
In total internal reflection, the angle of incidence must be greater than a certain minimum angle which depends on the media involved. |
| Statement II: |
Total internal reflection cannot occur when light is traveling from an optically rarer to an optically denser medium. |
| 1. |
Statement I is incorrect and Statement II is correct. |
| 2. |
Both Statement I and Statement II are correct. |
| 3. |
Both Statement I and Statement II are incorrect. |
| 4. |
Statement I is correct and Statement II is incorrect. |
184. Light, having a wavelength equal to the first line of the Balmer series, is incident onto a metal of work-function \(2\) eV. The kinetic energy of the ejected electron is:
1. \(1.4\) eV
2. \(0.5\) eV
3. \(0.1\) eV
4. no electrons are ejected
185. An electromagnetic waveform which has an
electric field given by:
\(\vec{E}=E_{0}[\hat{\imath} \cos (\omega t-k z)+\hat{\jmath} \cos (\omega t-k x)]\) and the waveform propagates. The maximum electric field has the magnitude:
| 1. |
\(\dfrac {E_0} { \sqrt 2}\) |
2. |
\(\sqrt 2~ E_0\) |
| 3. |
\(E_o\) |
4. |
\(2E_o\) |
Physics - Section B
186. Two identical simple pendulums are compared, one
\((A)\) located on the surface of the earth and the other
\((B)\) – at a
height \((h)\) above the earth's surface:
\(h=\dfrac{R}{1000}.\)
Their time periods are related as:
1.
\(T_A\Big(1+\dfrac{1}{1000}\Big)=T_B\)
2.
\(T_B\Big(1+\dfrac{1}{1000}\Big)=T_A\)
3.
\(T_A\Big(1+\dfrac{1}{2000}\Big)=T_B\)
4.
\(T_B\Big(1+\dfrac{1}{2000}\Big)=T_A\)
187. A long cylindrical solenoid of length
\(L\) and total number of turns
\(N\) produces a magnetic field
\(B_0\) at its centre with a current of
\(1\) A flowing through its coils. If the same current of
\(1\) A is sent through a circular wire of radius
\(R\) then the same field
\(B_0\) is produced at its centre. Then,
\(\dfrac{R}{L}\) equals:
| 1. |
\(N\) |
2. |
\(\dfrac 1N\) |
| 3. |
\(2N\) |
4. |
\(\dfrac{1}{2N}\) |
188. Two blocks of masses \(2m\), \(m\) are placed on a smooth horizontal table and they are in contact on their smooth slanted surfaces. A horizontal force \(F\), equal to \(mg\), is applied to the system from the left, which causes them to accelerate. Let \(N_A\) be the normal reaction from the table on \(A\), and \(N_B\) on \(B\). Then,

| 1. |
\(N_A = 2mg, N_B = mg\) |
| 2. |
\(N_A >2mg, N_B < mg\) |
| 3. |
\(N_A < 2mg, N_B > mg\) |
| 4. |
\(N_A < 2mg, N_B < mg\) |
189. A capacitor is being charged through a resistance, R. The capacitor was initially uncharged, and gets 75% charged in 20 s. If the capacitor is now allowed to discharge, it will lose 50 % of its initial charge in
(Take \(\Big(\frac{3}{4}\Big)^{2.4}\approx\frac12\), if required)
1. 20 s
2. 48 s
3. 10 s
4. 24 s
190. A uniform cylinder of mass
\(M,\) radius
\(R\) and height
\(3R\) is placed upright on a horizontal surface. A particle of mass
\(m\) is placed on the top of the cylinder at its edge. For what minimum value of
\(m\) will the cylinder topple?

| 1. |
\(m = 3M\) |
| 2. |
\(m= \dfrac {M}{3}\) |
| 3. |
\(m= \dfrac {3M }{2}\) |
| 4. |
No value of \(m\) will cause the cylinder to topple. |
191. A uniform rod of mass \(m\) and length \(L\) is struck at both ends by two particles of masses m, each moving with identical speeds \(u,\) but in opposite directions, perpendicular to its length. The particles stick to the rod after colliding with it. The system rotates with an angular speed:

| 1. |
\(\dfrac{u}{L}\) |
2. |
\(\dfrac{2u}{L}\) |
| 3. |
\(\dfrac{12u}{7L}\) |
4. |
\(\dfrac{6u}{L}\) |
192. A thermodynamic process involving a system takes place with
\(100~\text{J}\) of heat being rejected to the environment and an increase of
\(20~\text{J}\) of internal energy. Then,
| 1. |
work done by the system is \(120~\text{J}.\) |
| 2. |
work done on the system is \(120~\text{J}.\) |
| 3. |
work done by the system is \(80~\text{J}.\) |
| 4. |
work done on the system is \(80~\text{J}.\) |
193. In a certain region of space, equipotential surfaces of the electric field are drawn - corresponding to
\(V=10\) volt and \(V=9.9\) volts. There is no field along the
\(z\text-\)direction. At a certain point
\(P,\) on the
\(10-\)volt surface, the distance
\(PQ_1,\) to the
\(9.9\) volt surface is
\(2\) mm when
\(\overrightarrow{P Q}_{1}\) is along the
\(x\text-\)axis. On the other hand, if
\(\overrightarrow{P Q}_{2}\) is taken parallel to the
\(y\text-\)axis, the corresponding distance
\(PQ_2=1\) mm. The electric field at
\(P\) is along:
| 1. |
\(2 \hat{i}+\hat{j}\) |
2. |
\(2 \hat{j}+\hat{i}\) |
| 3. |
\(\dfrac{1}{4} \hat{i}+\hat{j}\) |
4. |
\( \dfrac{1}{4} \hat{j}+\hat{i}\) |
194. The self-inductance of a long solenoid of cross-section
\(A,\) total length
\(L\) and total number of turns
\(N,\) is (approximately):
| 1. |
\(\dfrac{\mu_0A}{L}\cdot N\) |
2. |
\(\dfrac{\mu_0A}{L}\cdot N^2\) |
| 3. |
\(\dfrac{\mu_0L^3}{A}\cdot N\) |
4. |
\(\dfrac{\mu_0L^3}{A}\cdot N^2\) |
195. A parallel beam of light is incident onto a converging lens
\((L_1)\) of focal length
\(20~\text{cm}.\) A second identical lens
\((L_2)\) is to be placed behind
\(L_1,\) coaxially, so that the emerging beam is parallel. The distance between
\(L_1\) and
\(L_2\) should be:

| 1. |
\(10\) cm |
2. |
\(20\) cm |
| 3. |
\(40\) cm |
4. |
zero |
196. Given below are two statements:
| Assertion (A): |
Water flows through a smooth horizontal tube with a narrowing cross-section and its pressure increases. |
| Reason (R): |
Bernoulli's equation for fluids states that \(P+\dfrac{1}{2}\rho v^2+\rho gh= \) constant along a streamline. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
197. Two particles
\(A,B\) move along the periphery of a circle of radius
\(R,\) with the same uniform speed
\(u.\) Particle
\(A\) follows
\(B,\) a quarter of the circumference behind it. The acceleration of
\(A\) relative to
\(B\) is:

| 1. |
zero |
2. |
\(\dfrac{2u^2}{R}\) |
| 3. |
\(\dfrac{u^2}{\sqrt2R}\) |
4. |
\(\dfrac{\sqrt2u^2}{R}\) |
198. The diode
\(D\) is ideal. The equivalent resistance between
\(A,B\) is:
\((V_A<V_B)\)
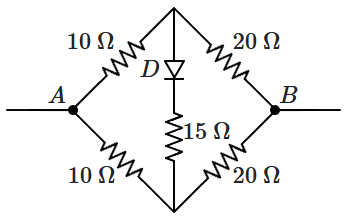
| 1. |
\(60~\Omega\) |
2. |
\(30~\Omega\) |
| 3. |
\(15~\Omega\) |
4. |
\(5~\Omega\) |
199. Two sources of sound vibrating at
\(200~\text{Hz}\) and
\(204~\text{Hz}\) are sounded together. The beat frequency heard is:
| 1. |
\(202~\text{Hz}\) |
2. |
\(404~\text{Hz}\) |
| 3. |
\(4~\text{Hz}\) |
4. |
\(2~\text{Hz}\) |
200. A force \(2x\hat i - 3y^2\hat j\) acts on a particle when it is at the location \(({x, y}).\) This force is:
| 1. |
non-conservative |
| 2. |
conservative and the potential energy is \(({x^2-y^3})\) |
| 3. |
conservative and the potential energy is \(({y^3-x^2})\) |
| 4. |
conservative, but it cannot have a potential energy |
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course







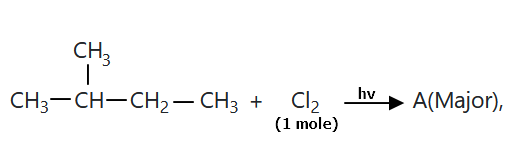
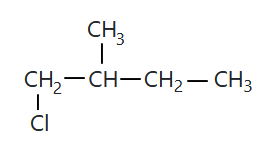
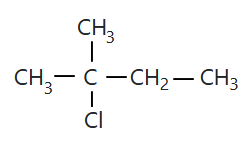
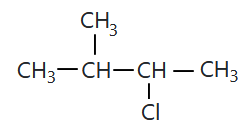
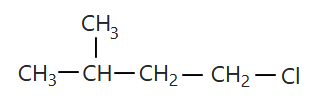
 1. zero
1. zero


















