Hairy leaves in several plants are not associated with resistance to insect pests in:
1. Resistance to jassids in cotton
2. Cereal leaf beetle in wheat
3. Resistance to aphids in brassica
4. Both 2 and 3
Natural reservoir of phosphorus is:
1. Animal bones
2. Rock
3. Fossils
4. Sea water
Who proposed semiconservative mode of replication in DNA?
1. Meselson and Stahl
2. Watson and Crick
3. Calm
4. Taylor
Which of the following is incorrect statement with respect to carbohydrates?
| 1. | Mannose and galactose are polymers of glucose |
| 2. | Cellulose acetate is used in fabrics, cellulosic plastics and shatter proof glasses |
| 3. | Most of sugars show Benedict's test but not sucrose |
| 4. | Sucrose is composed of glucose and fructose and is a non-reducing sugar |
|
A |
B |
C |
 |
 |
 |
Identify gametes (A, B and C) respectively:
| 1. | Heterogametes, isogametes, Homogametes |
| 2. | Isogametes, homogametes, heterogametes |
| 3. | Homogametes, isogametes, heterogametes |
| 4. | Homo/Isogametes, heterogametes, heterogametes |
Which one of the following group of plants do not show the clear cut distinction of vegetative, reproductive, and senescent phases?
1. Annuals
2. Monocarpic biennials
3. Perennials
4. More than one option is correct
Match the following
| (I) | External fertilization | (i) | pollen grain |
| (II) | Androecium | (ii) | anther wall |
| (III) | Male gametophyte | (iii) | algae |
| (IV) | Primary parietal layer | (iv) | stamens |
1. I-iv;II-i;III-ii;IV-iii
2. I-iii;II-iv;III-i;IV-ii
3. I-iii;II-iv;III-ii,IV-i
4. I-iii;II-i;III-iv;IV-ii
Which of the following statements are true related to Seed X and Y?
| (i) | Seed X is dicot and endospermic or albuminous. |
| (ii) | Seed X is dicot and non-endospermic or non-albuminous. |
| (iii) | Seed Y is monocot and endospermic or albuminous. |
| (iv) | Seed Y is monocot and non-endospermic or non-albuminous. |
Choose the correct option with the respect to the nature of the seed
| 1. | (i), (iii) | 2. | (ii), (iii) |
| 3. | (i), (iv) | 4. | (ii), (iv) |
Select the incorrect statement:
| 1. | Isolated metabolic reactions in vitro are living reactions |
| 2. | Human beings are the only organism to have self-consciousness |
| 3. | Reproduction is an all-inclusive property of living beings |
| 4. | Unicellular organisms grow by cell-division |
| 1. | Amyloplast | (i) | Store protein granules |
| 2. | Elaioplast | (ii) | Store oils or fats |
| 3. | Chloroplasts | (iii) | Contain chlorophyll pigments |
| 4. | Chromoplasts | (iv) | Contains colored pigments other than chlorophyll |
According to the five-kingdom classification system, which of the following kingdom has multicellular/loose tissue level body organization?
1. Protista
2. Plantae
3. Animalia
4. Fungi
Match the Column-l with Column-ll
| Column-l | Column-ll | ||
| (a) | Marginal placentation | (i) | Single ovule is present in unilocular ovary |
| (b) | Axile placentation | (ii) | False septum may be present |
| (c) | Parietal placentation | (iii) | Placenta forms a ridge along the ventral suture of ovary |
| (d) | Basal placentation | (iv) | Tomato, Lemon |
| (a) | (b) | (c) | (d) | |
| 1. | (iii) | (i) | (iv) | (ii) |
| 2. | (ii) | (iii) | (iv) | (i) |
| 3. | (iii) | (iv) | (ii) | (i) |
| 4. | (iv) | (iii) | (ii) | (i) |
Study the diagram given below and choose the correct information that can be deduced :
| 1. | The core RNA polymerase is capable of catalyzing elongation only |
| 2. | Sigma factor and Rho factor are needed for termination of transcription |
| 3. | DNA helicase opens the strand of the DNA |
| 4. | Both strands of DNA are transcribed by the same RNA polymerase |
Mendel’s work remained unrecognized till 1900. Which of the following was not a reason for this?
| 1. | His work was widely publicized and it brought bad name to Mendel. |
| 2. | His concept of factors as stable and discrete units that did not ‘blend’ was not accepted. |
| 3. | His approach of using mathematics to explain biological phenomena was unacceptable. |
| 4. | He could not provide any physical proof for the existence of factors or say what they were made of. |
| Column-I | Column-II | ||
| I. | Operator site | (A) | Binding site for RNA polymerase |
| II. | Promoter site | (B) | Binding site for repressor molecule |
| III. | Structural gene | (C) | Codes for enzyme protein |
| IV. | Regulator gene | (D) | Code for repressor molecules |
The correct match is:
| I | II | III | IV | |
| 1. | B | A | C | D |
| 2. | B | A | D | C |
| 3. | D | C | A | B |
| 4. | B | C | A | D |
The dough kept overnight in warm weather becomes soft and spongy because of:-
1. Fermentation
2. Cohesion
3. Osmosis
4. Absorption of carbon dioxide from the atmosphere
There are five factors known to affect Hardy-Weinberg equilibrium. Which of the following cannot be included in the five factors?
1. Gene flow and genetic drift
2. Genetic recombination and natural selection
3. Mutation
4. Isolation
Which of the following statements is wrong?
| 1. | There are techniques that are able to detect the minerals even at a very low concentration i.e. 10-8 g/mL |
| 2. | Some plant species accumulate selenium while some others gold |
| 3. | Some plants growing near nuclear test sites take up radioactive strontium |
| 4. | The criteria for essentiality of an element completely depends upon the amount of element |
Read the statements a, b, c, and d and select the correct choice with respect to gibberellins: (GAs)
| a. | They delay senescence |
| b. | Promote bolting just prior to vegetative growth |
| c. | They are isolated from fungi only |
| d. | Induce flowering in LDP |
| 1. | a, b & c are correct |
| 2. | b, c & d are incorrect |
| 3. | a & d are correct |
| 4. | b, c & d are correct |
Biolistics (gene-gun) is suitable for:
| 1. | Disarming pathogen vectors |
| 2. | Transformation of plant cells |
| 3. | Constructing recombinant DNA by joining with vectors |
| 4. | DNA finger printing |
In which of the following do both pairs have the correct combination?
1. In situ conservation: National Park
Ex situ conservation: Botanical Garden
2. In situ conservation: Cryopreservation
Ex situ conservation: Wildlife Sanctuary
3. In situ conservation: Seed Bank
Ex situ conservation: National park
4. In situ conservation: Tissue culture
Ex situ conservation: Sacred groves
Which of the following is a correct pair?
| 1. | Cuscuta – parasite | 2. | Dischidia – insectivorous |
| 3. | Opuntia – predator | 4. | Capsella – hydrophyte |
Transpiration is very important for plants because it helps in:
1. the absorption of water from soil
2. the cooling of leaves at high temperature
3. the movement of water and minerals absorbed by roots to various parts of the plant
4. all of the above
Which of the following statement is incorrect?
| 1. | Oxygen is released from water |
| 2. | Photosynthesis is a single step reaction |
| 3. | Action spectrum of photosynthesis resembles roughly the absorption spectra of chlorophyll a and b |
| 4. | In plants glucose is stored as starch |
Complex II has the following components except:
| 1. | FAD | 2. | Fe-S centres |
| 3. | Succinate Dehydrogenase | 4. | Cytochrome C1 |
The diagram given below belongs to -
| 1. | Dicot stem having conjoint and collateral vascular bundles |
| 2. | Monocot stem having conjoint and collateral vascular bundles |
| 3. | Dicot root with polyarch condition |
| 4. | Monocot root with radial vascular bundles |
Asparagus comes under which category?
1. Colchicine
2. Vegetables
3. Ornaments
4. Medicine
Study the figure and find out an incorrect statement:
| 1. | Contains laminarin or mannitol as reserve food. |
| 2. | Life-cycle is diplontic. |
| 3. | Union of gamete may take place in water or within the oogonium. |
| 4. | Gametes bear two apically inserted flagella. |
The non-cellular organisms that are characterized by having inert crystalline structures outside the living cell:
1. Were discovered by J. Pasteur
2. Are obligate intercellular parasites
3. Have genetic material as either RNA or DNA
4. Are cellular organisms
Xerarch and Hydrarch succession leads to which type of respective climax communities?
1. Xeric and hydric
2. Hydric and xeric
3. Mesic and mesic
4. Xeric and mesic
The length of DNA molecule greatly exceeds the dimensions of the nucleus in eukaryotic cells. How is this DNA accommodated?
1. Deletion of non-essential genes
2. Super-coiling in nucleosomes
3. DNAse digestion
4. Through elimination of repetitive DNA
All of the following statements about Hardy-Weinberg equilibrium are correct except:
| 1. | Any change in Hardy-Weinberg equilibrium indicates evolution |
| 2. | Gene flow, genetic drift, mutation, genetic recombination, and natural selection are five factors which affect this equilibrium |
| 3. | Random mating also induces a change in this equilibrium |
| 4. | Hardy-Weinberg equilibrium is a tool to measure the extent of evolutionary change |
| 1. | Heterozygous | 2. | Homozygous |
| 3. | Monohybrid | 4. | Non-identical |
Which one of the following statements regarding post-fertilization development in flowering plants is incorrect?
1. Ovules develop into embryo sac
2. Ovary develops into fruit
3. Zygote develops into embryo
4. Central cell develops into endosperm
DNA dependent RNA polymerase catalyzes polymerization in:
1. Only in 3’ – 5’ direction
2. Only in 5’ – 3’ direction
3. In both directions
4. In neither directions
Which of the following statements is correct?
| 1. | Organisms that depend on living plants are called saprophytes. |
| 2. | Some of the organisms can fix atmospheric nitrogen in specialized cells called sheath cells. |
| 3. | The fusion of two cells is called Karyogamy. |
| 4. | Fusion of protoplasms between two motile on non-motile gametes is called plasmogamy. |
Which of the following are not secondary metabolites in plants?
1. Vinblastin, curcumin
2. Rubber, gums
3. Morphine, codeine
4. Amino acids, glucose
At the root apex the cells of the meristematic region are not characterized by:-
1. Being rich in protoplasm
2. Abundant plasmodesmatal connections
3. A cell wall that is primary in nature
4. Having small inconspicuous nuclei
What functions as the embryonic root of the plant?
| 1. | A | 2. | B |
| 3. | C | 4. | D |
Which of the following statement is not true about the family Liliaceae?
| 1. | Perennial herbs with underground bulbs/corms/rhizomes |
| 2. | Flower: bisexual, zygomorphic |
| 3. | Gynoecium: tricarpellary, syncarpous, superior, trilocular with many ovules, axile placentation |
| 4. | Tulip, Gloriosa, Aloe, Asparagus, and colchicum belongs to Liliaceae |
The Stomatal apparatus comprises:
1. stomatal aperture and guard cells
2. stomatal aperture, guard, and subsidiary cells
3. stomatal aperture, guard, subsidiary, and epidermal cells
4. stomatal aperture and subsidiary cells
From the characters given below, how many of them are associated with numerical taxonomy?
| i. | No usage of computers for data analysis |
| ii. | Based on all observable characteristics |
| iii. | After the data processing, numbers and codes are assigned to all characters |
| iv. | Hundreds of characters are considered at the same time |
| v. | Give equal weightage to all characters |
Choose the correct option:
1. 5
2. 4
3. 3
4. 2
Most of the minerals are absorbed through active transport because:
1. Minerals are charged particles
2. Minerals are insoluble in lipid bilayer
3. The amount of minerals is less in the soil
4. All of these
The ________ accepter of ______ which is located towards the ____ side of the membrane transfers its electron not to an electron carrier but to an _____. Fill in the blank according to the causes of the proton gradient in photosynthesis:
| 1. | Primary, proton, inner, proton carrier |
| 2. | Primary, electron, outer, H carrier |
| 3. | Terminal, proton, outer, proton carrier |
| 4. | Primary, electron, inner, H carrier |
Which one of the following is an integral membrane protein complex that forms the channel through which the protons cross the inner membrane?
1. \(F_0-F_1\)
2. \(F_0\)
3. \(F^+\) and \(F^-\)
4. \(CF_1\)
In an electrostatic precipitator:
| 1. | The velocity of air between the plates must be low enough to allow the dust to fall. |
| 2. | The velocity of air between the plates must be very high to purify the air. |
| 3. | There is no air between plates so that the charge may not get disturbed. |
| 4. | None of these |
Which of the following is true about primary metabolites?
| 1. | Compounds which are products of essential pathways of plants |
| 2. | Universally present in all plant kingdom |
| 3. | Its absence leads to the death of plant cell |
| 4. | All of the above |
Which of the following option is wrong about Water hyacinth?
1. World's most problematic weed
2. Terror of Bengal
3. Grow abundantly in eutrophic water bodies
4. Maintains a balance in the ecosystem dynamics of the water body
Match the following events that occur in their respective phases of cell cycle and select the correct option :
| (a) G1 phase | (i) Cell grows and organelle duplication |
| (b) S phase | (ii) DNA replication |
| (c) G2 phase | (iii) Cytoplasmic growth |
| (d) Metaphase in M-phase | (iv) Alignment of chromosomes |
| (a) | (b) | (c) | (d) | |
| 1. | (ii) | (iii) | (iv) | (i) |
| 2. | (iii) | (iv) | (i) | (ii) |
| 3. | (iv) | (i) | (ii) | (iii) |
| 4. | (i) | (ii) | (iii) | (iv) |
A certain road accident patient with unknown blood group needs immediate blood transfusion. His one doctor friend at once offers his blood. What was the blood group of the donor?
1. Blood group B
2. Blood group AB
3. Blood group O
4. Blood group A
Defecation is not:
1. a voluntary process
2. carried out by mass-peristaltic movement
3. initiated by a local reflex
4. All the above options are wrong with respect to the asked question
Diffusion membrane consists of:
1. Squamous epithelium of alveoli
2. endothelium of alveolar capillaries
3. Basement substance between them
4. All of the above
Find the odd statement:
| 1. | SA node generates the maximum number of action potential per minute so it sets the pace of the activities of the heart |
| 2. | About 70 ml of blood pumped out by each ventricle during a cardiac cycle is stroke volume |
| 3. | Heart is autoexcitable, its function can be moderated by neural and hormonal mechanisms |
| 4. | Systemic heart includes the right ventricles and left atrium |
Disc-shaped proteinaceous structure attached to centromere of a chromosome is called?
1. Chromocentre
2. NOR
3. Chromomere
4. Kinetochore
Which of the following is correct?
| 1. | The ES complex formation is a transient phenomenon. |
| 2. | The structure of the substrate gets transformed into the structure of product. |
| 3. | All the structures formed between substrate and product are called intermediates. |
| 4. | All of these |
Different types of hearts and the blood circulation patterns seen in the animal kingdom are given in the following table. Which of the following is an incorrect match with respect to the animal group and types of heart?
|
Animal group |
Type of heart |
|
|
1. |
Fishes |
Two chambered |
|
2. |
Amphibians |
Three Chambered |
|
3. |
Birds |
Incompletely four chambered |
|
4. |
Mammals |
Four chambered |
Study the pedigree chart given below.
What does it show?
| 1. | Inheritance of a sex-linked inborn error of metabolism |
| 2. | Inheritance of a condition like phenylketonuria as an autosomal recessive trait |
| 3. | The pedigree chart is wrong as this is not possible |
| 4. | Inheritance of a recessive sex-linked disease like haemophilia |
Embryological support for evolution was proposed by
1. Ernst Heckel
2. Karl Ernst von Baer
3. Charles Darwin
4. Alfred Wallace
The JG cells are activated by:
1. A fall in Glomerular blood flow
2. A fall in Glomerular blood pressure
3. A fall in GFR
4. All of these
Which of the following is true regarding photoreceptor cells?
1. Have light-sensitive glycoproteins
2. Photopic vision is a function of rods
3. Scotopic vision is a function of cones
4. Color vision is a function of cones
Which of the following sex hormones produces anabolic synthetic effects on protein and carbohydrate metabolism?
1. Testosterone
2. Estrogen
3. Progesterone
4. Relaxin
| 1. | It is mesodermal in origin |
| 2. | 40-50% of body weight is formed by it |
| 3. | They have properties like excitability and contractibility |
| 4. | They are classified on the basis of reception of a type of stimulus |
MOET has not been practiced in:
a. Cattle b. Sheep
c. Rabbits d. Poultry
1. b,c & d
2. b & d
3. d only
4. c only
The colonies of recombinant bacteria appear white in contrast to blue colonies of non-recombinant bacteria because of:
| 1. | Insertional inactivation of alpha-galactosidase in non-recombinant bacteria |
| 2. | Insertional inactivation of beta-galactosidase in recombinant bacteria |
| 3. | Inactivation of galactase enzyme in recombinant bacteria |
| 4. | Recombinant bacteria containing beta-galactosidase |
Which body of the Government of India regulates GM research and safety of introducing GMO for public services?
| 1. | Indian Council of Agricultural Research |
| 2. | Genetic Engineering Approval Committee |
| 3. | Research Committee on Genetic Manipulation |
| 4. | Bio-safety committee |
The process of separation and purification of expressed protein before marketing is called?
1. Upstream processing
2. Downstream processing
3. Bioprocessing
4. Postproduction processing
An enzyme catalysing the removal of nucleotides from ends of DNA is:
| 1. | DNA ligase | 2. | Endonuclease |
| 3. | Exonuclease | 4. | Protease |
What is antisense technology?
| 1. | A cell displaying a foreign antigen used for synthesis of antibodies |
| 2. | Production of somaclonal variants in tissue culture |
| 3. | A complimentary RNA used to stop expression of a specific gene |
| 4. | RNA polymerase producing DNA |
Cell theory which was earlier proposed by Schleiden and Schwann, failed to explain:
1. how new cells are formed
2. how cells die
3. how cell division takes place
4. how a plant cell is different from an animal cell
Find the wrong match:
1. Churning movement- stomach
2. Chyme- stomach
3. Bolus- mouth
4. Stomach- storage of food for 2 to 3 hrs
Which of the following substances are not reabsorbed by active means of transport?
1. Glucose
2. Amino Acids
3. Nitrogenous waste
4. Na+
Which of the following situations would result in the greatest degree of saturation for haemoglobin, assuming remains constant-
1. Increased levels, decreased temperature.
2. Increased levels, increased acidity.
3. Decreased levels, decreased acidity.
4. Increased levels, increased temperature.
Which of the following is true about estrogen?
1. responsible for high pitch of voice
2. regulates female sexual behaviour
3. regulates growth of female secondary sex organs
4. All of the above
Which amongst the following actions cannot be classified as simple reflex actions?
| 1. | Dancing | 2. | Knee jerk |
| 3. | Blinking | 4. | Coughing |
The life span of the erythrocytes in humans is about:
1. 120 days
2. 150 days
3. 190 days
4. 180 days
Many elements are found in living organisms either free or in the form of compounds. Which one of the following is not found in living organisms?
| 1. | Silicon | 2. | Magnesium |
| 3. | Iron | 4. | Sodium |
Which of the following microbes is correctly paired with its function ?
1. Aspergillus niger - Production of lactic acid
2. Trichoderma polysporum - Lowers blood cholesterol
3. Saccharomyces cerevisiae - Production of citric acid
4. Methanogenic bacteria - Gobar gas formation
During menstrual cycle, levels of LH and estrogen are highest around:
1. 14th day
2. 21st day
3. 7th day
4. 28th day
Which of the following doesn't hold true for adolescence?
| 1. | Accompanied by many biological and behavioral changes |
| 2. | Vulnerable phase of mental and psychological development of an individual |
| 3. | A child becomes mature for marriage and becomes effective in education |
| 4. | The period between 12-18 years of age |
According to A mutation was gradual while de Vries believed mutation caused B and hence called it C
| A | B | C | |
| 1. | Lamarck | Saltation | Speciation |
| 2. | Thomas Malthus | Speciation | Natural selection |
| 3. | Darwin | Speciation | Saltation |
| 4. | Wallance | Natural selection | Speciation |
A couple is unable to conceive for many years. It is found out that the wife is healthy and produces normal ova. The husband however has low sperm count. They are enthusiastic about having a child using ART but are adamant that the child should genetically be theirs. The procedures X and Y can be used by the couple.
| i. | The wife’s ovum is retrieved and so are the husband’s sperms, and they are made to fertilise outside the body-X. |
| ii. | Embryo of more than 8 blastomeres is transferred in the wife-Y. |
Select the correct procedures for steps X and Y from the following:
IUT, IUD, GIFT, ZIFT, IUI, ICSI, AI, IVF, in vivo fertilization
1. X: in vivo fertilisation; Y: ZIFT
2. X: IVF; Y: GIFT
3. X: in vivo fertilisation; Y: ICSI
4. X: IVF; Y: IUT
| A | B | |
| 1. | Ethidium bromide | UV radiations |
| 2. | Bromophenol blue | UV radiations |
| 3. | Ethidium bromide | Visible light |
| 4. | Methylene blue | Visible light |
Diagrams (A, B, C and D) of four different animals are given below. Which one of the following option identifies the animal, its phylum and one of its characters correctly ?
| Animal | Phylum | Character | |
| B | Rana | Amphibia | 3 – chambered heart |
| C | Asterias | Echinodermata | Water canal system |
| A | Aurelia | Coelenterata | Cnidoblast cells |
| D | Naja | Chordata | Non-poisonous snake |
1. B
2. C
3. A
4. D
Select the correct sequence for transport of sperm cells in male reproductive system.
| 1. | Testis → Epididymis → Vasa efferentia → Vas deferens → Ejaculatory duct → Inguinal canal → Urethra → Urethral meatus |
| 2. | Testis → Epididymis → Vasa efferentia → Rete testis → Inguinal canal → Urethra |
| 3. | Seminiferous tubules → Rete testis → Vesa efferentia → Epididymus → Vas deferens → Ejaculatory duct → Urethra → Urethral meatus |
| 4. | Seminiferous tubules → Vasa efferentia → Epididymis → Inguinal canal → Urethra |
When any plane of section passing through the central axis of the body divides the organism into identical halves it is called?
| 1. | Radial symmetry |
| 2. | Biradial symmetry |
| 3. | Bilateral symmetry |
| 4. | Spherical symmetry |
Tricuspid Valves are present between:
1. left atria and aorta
2. right atria and right ventricle
3. left atria and left ventricle
4. both 1 and 2
The solubility of carbon dioxide is about ...........times higher than that of oxygen across the respiratory membrane.
| 1. | 20-25 | 2. | 25-50 |
| 3. | 125-150 | 4. | 200-250 |
The lining of PCT is made up of:
1. Simple cuboidal brush border epithelium
2. Simple squamous brush border epithelium
3. Simple columnar brush border epithelium
4. Compound cuboidal brush border epithelium
For the attachment of 8th, 9th and 10th ribs, which of the following cartilages are found?
| 1. | Hyaline | 2. | Elastic |
| 3. | Fibroelastic | 4. | Reticular |
Which of the following is incorrect about adrenal gland?
1. Located on posterior part of kidney
2. located above kidney
3. 2 in number
4. composed of 2 types of tissues
The major coordinating centre for sensory and motor signaling is __X__ and centre for controlling respiration, cardiovascular reflex and gastric secretion is __Y__.
| X | Y | |
| 1. | Medulla Oblongata | Cerebrum |
| 2. | Cerebrum | Pons |
| 3. | Thalamus | Medulla Oblongata |
| 4. | Hypothalamus | Pons |
Trace the path of physiologically mature sperm in the male reproductive tract:
| 1. | Seminiferous tubules → Rete testis → Vasa efferentia → Epididymis → Vas deferens → Ejaculatory duct → urethra |
| 2. | Vas deferens → Epididymis → Ejaculatory duct → Urethra |
| 3. | Rete testis → Epididymis → Vasa efferentia → Vas deferens → Ejaculatory duct → urethra |
| 4. | Epididymis → Ejaculatory duct → Vas deference → Urethra |
For which of the following is the number same in spermatogenesis and oogenesis?
1. lnterruptions in the meiotic division
2. Functional gametes produced by meiosis
3. Meiotic divisions required to produce each gamete
4. Gametes produced in a given period
Veneral diseases can spread through:
| (a) | Using sterile needles |
| (b) | Transfusion of blood from infected person |
| (c) | Infected mother to foetus |
| (d) | Kissing |
| (e) | Inheritance |
Choose the correct answer from the options given below.
1. (b) and (c) only
2. (a) and (c) only
3. (a), (b) and (c)
4. (b), (c) and (d)
Which of the following options gives the correct sequence of events during mitosis?
| 1. | Condensation, nuclear membrane disassembly, arrangement at equator, centromere division, segregation, telophase |
| 2. | Condensation, crossing over, nuclear membrane disassembly, segregation, telophase |
| 3. | Condensation, centromere division, segregation, arrangement at equator, telophase |
| 4. | Condensation, nuclear membrane disassembly, crossing over, segregation, telophase |
The theory of special creation was strongly challenged during the nineteenth century on the basis of observations made:
| 1. | During a sea voyage in a sailing ship called H.M.S. Beagle around Europe |
| 2. | During a sea voyage in a sailing ship called M.H.S. Beagle around Africa |
| 3. | During a sea voyage in a sailing ship called H.M.S. Beagle around the world |
| 4. | During a sea voyage in a sailing ship called M.H.S. Beagle around the world |
Which of the following techniques is used to detect the cancer of internal organs?
| 1. | Radiography |
| 2. | Computerized tomography |
| 3. | Magnetic resonance imaging |
| 4. | All of these |
A and B in the pBR 322, shown in the diagram given below, respectively represent recognition sequences of :
| 1. | BamH I and Sma I | 2. | Hind II and Sma I |
| 3. | BamH I and SaI I | 4. | SaI I and Hind II |
What type of human population is represented by the following age pyramid?
| 1. | Expanding population | 2. | Vanishing population |
| 3. | Stable population | 4. | Declining population |
Mixture of chloroxylenol and terpineol acts as:
1. Antiseptic
2. Antipyretic
3. Antibiotic
4. Analgesic
Equanil is used as:
1. Analgesic
2. Antibiotic
3. Tranquilizer
4. Antacid
Cetyltrimethyl ammonium bromide is an example of :
1. Artificial sweetener
2. Cationic detergent
3. Soap
4. Anionic detergent
Plastic household crockery is prepared by using :
1. Melamine and tetrafluoroethane.
2. Malonic acid and hexamethylene imine.
3. Melamine and vinyl acetate.
4. Melamine and formaldehyde.
Which of the following oxides behaves as a conductor or insulator depending upon temperature?
1. TiO
2. SiO2
3. TiO3
4. MgO
The correct statements among the following are:
| a. | Micelle formation by soap in an aqueous solution is possible at all temperatures |
| b. | Micelle formation by soap in an aqueous solution occurs above a particular concentration |
| 3. | On dilution of soap solution micelles may revert to individual ions |
| 4. | Soap solution behaves like a normal strong electrolyte at all concentrations |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
In the extraction of copper from its sulphide ore, the metal is formed by the reduction of with :
1.
2.
3.
4.
Among [Ni(CO)4], [Ni(CN)4]2−, [ NiCl4]2− species, the hybridization states of the Ni atom are respectively:
(At. No. of Ni = 28)
| 1. | sp3, dsp2, sp3 | 2. | sp3, sp3, dsp2 |
| 3. | dsp2, sp3, sp3 | 4. | sp3, dsp2 , dsp2 |
Choose the incorrect statement among the following regarding noble gases:
| 1. | Noble gases have weak dispersion forces. |
| 2. | Noble gases have large positive values of electron gain enthalpy. |
| 3. | Noble gases are sparingly soluble in water. |
| 4. | Noble gases have very high melting and boiling points. |
A balloon is filled with hydrogen at room temperature. It will burst if pressure exceeds 0.2 bar. If at 1 bar pressure the gas occupies 2.27 L volume, upto what volume can the balloon be expanded ?
1. 11.35 L
2. 08.35 L
3. 13.35 L
4. None of the above
The number of chiral carbons present in the molecule given below is:
1. 8
2. 7
3. 5
4. 6
The number of subshells associated with n = 4 and m = –2 quantum numbers is:
1. 4
2. 8
3. 16
4. 2
The volume strength of 8.9 M H2O2 solution calculated at 273 K and 1 atm is-
(R = 0.0821 L atm K–1 mol–1) rounded off to the nearest integer)
1. 100
2. 80
3. 120
4. 60
[P] on treatment with Br2/FeBr3 in CCl4 produced a single isomer C8H7O2Br while heating [P] with soda lime gave toluene. The compound [P] is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The processes of calcination and roasting in metallurgical industries, respectively, can lead to :
| 1. | Global warming and acid rain. |
| 2. | Photochemical smog and global warming. |
| 3. | Photochemical smog and ozone layer depletion. |
| 4. | Global warming and photochemical smog. |
Among the statements (I – IV), the correct ones are:
| (I) | Be has a smaller atomic radius compared to Mg. |
| (II) | Be has higher ionization enthalpy than Al. |
| (III) | Charge/radius ratio of Be2+ is greater than that of Al3+. |
| (IV) | Both Be and Al form mainly covalent compounds. |
1. (I), (II), and (III)
2. (I), (II), and (IV)
3. (I), (III), and (IV)
4. (II), (III), and (IV)
Consider the following compounds:
BeO, BaO, Be(OH)2, Sr(OH)2
The amphoteric compound(s) are:
| 1. | BeO, BaO | 2. | BaO, Be(OH)2 |
| 3. | Be(OH)2, BeO | 4. | Sr(OH)2, Be(OH)2 |
The reaction of sulphur in an alkaline medium is given below:
The value of 'a' is:
| 1. | 10 | 2. | 12 |
| 3. | 14 | 4. | 17 |
The stepwise formation of [Cu(NH3)4]2+ is given below
The value of stability constants K1, K2, K3 and K4 respectively. The overall equilibrium constants for the dissociation of
(Rounded off to the nearest integer)
| 1. | 2 | 2. | 4 |
| 3. | 3 | 4. | 1 |
The major product 'Z' formed in the following reaction is: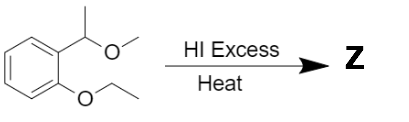
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Given that the equilibrium constant (KC) at 800 K for the reaction N2(𝑔)+3H2(𝑔)⇋2NH3(𝑔) is 64. What is the equilibrium constant KC at the same temperature for the reaction NH3(g) ⇌ 1/2N2(g) + 3/2H2(g)?
| 1. | \(\dfrac{1}{4}\) | 2. | \(\dfrac{1}{8}\) |
| 3. | 8 | 4. | \(\dfrac{1}{64}\) |
A set of solutions is prepared using 180 g of water as a solvent and 10 g of different non-volatile solutes A, B and C. The relative lowering of vapour pressure in the presence of these solutes are in the order:
[Given, molar mass of A = 100 g ; B = 200 g ; C = 10,000 g ]
| 1. | A > B > C | 2. | A > C > B |
| 3. | C > B > A | 4. | B > C > A |
The number of significant figures in 50000.020 × 10–3 is:
| 1. | 11 | 2. | 5 |
| 3. | 8 | 4. | 4 |
If dichromate ion is treated with base, the oxidation number of Cr in the product formed will be:
1. +6
2. +4
3. +5
4. +2
For the reaction, the plot of log k vs is given below :
Find the temperature(K) at which the rate constant of the reaction is 10–4s–1 ?
(Rounded-off to the nearest integer)
[Given: The rate constant of the reaction is
| 1. | 546 K | 2. | 536 K |
| 3. | 516 K | 4. | 526 K |
The structure of IF7 is :
1. Square pyramidal
2. Trigonal bipyramidal
3. Octahedral
4. Pentagonal bipyramidal
For a particular reversible reaction at temperature T, ∆H and ∆S were found to be both +ve. If Te is the temperature at equilibrium, the reaction would be spontaneous when:
1. T = Te
2. Te > T
3. T > Te
4. Te is 5 times T
In view of the signs of for the following reactions:
The oxidation states more characteristic for lead and tin are respectively:
1. For lead +2, for tin +2
2. For lead +4, for tin +4
3. For lead +2, for tin +4
4. For lead +4, for tin +2
Resistance of 0.2 M solution of an electrolyte is 50 . The specific conductance of the solution is 1.3 S m-1. If the resistance of the 0.4 M solution of the same electrolyte is \(260 \Omega,\) its molar conductivity is:
1.
2.
3.
4.
In the reaction
Identify 𝐶:
1. C6H5OH
2. C6H6
3. C6H5COONa
4. C6H5ONa
| 1. |  |
| 2. | |
| 3. | |
| 4. |
The molecules/ions that do not contain unpaired electrons among the following is:
1.
2. B2
3.
4. O2
In a closed reaction vessel, Phosphorus pentachloride dissociates as follows:
\(\mathrm{PCl}_{5}(g) \rightleftharpoons \mathrm{PCl}_{3}(g)+\mathrm{Cl}_{2}(g)\)
If the total pressure at equilibrium of the reaction mixture is P and the degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be:
1. \(\left(\frac{x}{x+1}\right) P\)
2. \(\left(\frac{2x}{x-1}\right) P\)
3. \(\left(\frac{x}{x-1}\right) P\)
4. \(\left(\frac{x}{1-x}\right) P\)
The IUPAC name of the following compound is:

1. 3, 3-Dimethyl-1-hydroxycyclohexane
2. 1,1-Dimethyl-3-hydroxycyclohexane
3. 3,3-Dimethyl-1-cyclohexanol
4. 1,1-Dimethyl-3-cyclohexanol
Iron carbonyl, , is classified as:
| 1. | Tetranuclear | 2. | Mononuclear |
| 3. | Trinuclear | 4. | Dinuclear |
| 1. | 17.6 mg | 2. | 21.3 mg |
| 3. | 24.3 mg | 4. | 13.6 mg |
The structure of intermediate A in the following reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The graphs that represent a zero-order reaction are:
| (a) |  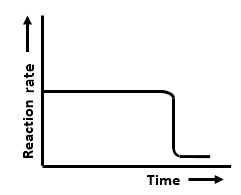 |
(b) |  |
| (c) |  |
(d) |  |
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |
Addition of water to alkynes occurs in acidic medium and in the presence of ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The mole ratio of H2 and O2 gas is 8:1. The ratio of their weight will be:
| 1. | 1 : 1 | 2. | 2 : 1 |
| 3. | 4 : 1 | 4. | 1 : 2 |
The incorrect statements among the following are-
| (a) | NaCl being an ionic compound is a good conductor of electricity in the solid-state |
| (b) | In canonical structure, there is a difference in the arrangement of atoms |
| (c) | Hybrid orbitals form stronger bonds than pure orbitals |
| (d) | VSEPR theory can explain the square planar geometry of XeF4 |
| 1. | (a) and (b) only | 2. | (b) and (c) only |
| 3. | (c) and (d) only | 4. | (b) and (d) only |
The vapour pressures of pure liquids A and B are 400 and 600 mm Hg, respectively, at 298 K. On mixing the two liquids, the sum of their initial volumes is equal to the volume of the final mixture. The mole fraction of liquid B is 0.5 in the mixture. The vapour pressure of the final solution, the mole fractions of components A and B in the vapour phase, respectively, are:
1. 500 mm Hg, 0.5, 0.5
2. 450 mm Hg, 0.5, 0.5
3. 500 mm Hg, 0.4, 0.6
4. 450 mm Hg, 0.4, 0.6
The molar solubility of Cd(OH)2 is 1.84 × 10–5 M in water. The expected solubility of Cd(OH)2 in a buffer solution of pH = 12 is:
| 1. | \(2.49 \times 10^{-10} M\) | 2. | \(1.84 \times 10^{-9} M\) |
| 3. | \(6.23 \times 10^{-11} M\) | 4. | \(1.49 \times 10^{-9} M\) |
Consider the following molecules and statements related to them:

| (I) | (B) is more likely to be crystalline than (A) |
| (II) | (B) has a higher boiling point than (A) |
| (III) | (B) dissolves more readily than (A) in water |
Identify the correct option from below :
1. (I) and (II) are false
2. Only (III) are true
3. Only (I) is true
4. (I), (II) and (III) are true
The lanthanoid that does not show +4 oxidation state is:
| 1. | Dysprosium (Dy) | 2. | Europium (Eu) |
| 3. | Cerium (Ce) | 4. | Terbium (Tb) |
Identify A in the given chemical reaction:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
| Statement I: | A mixture of chloroform and aniline can be separated by simple distillation. |
| Statement II: | When separating aniline from a mixture of aniline and water by steam distillation aniline boils below its boiling point. |
The correct option is:
| 1. | Statement I is false but Statement II is true. |
| 2. | Both Statement I and Statement II are false. |
| 3. | Statement I is true but Statement II is false. |
| 4. | Both Statement I and Statement II are true. |
Consider the following carbanions:
| (I) |  |
| (II) |  |
| (III) |  |
| 1. | I > II > III | 2. | III > II > I |
| 3. | II > III > I | 4. | I > III > II |
If the same amount of heat is supplied to two spheres of the same material having the same radius (one is hollow and the other is solid), then:
| 1. | the expansion in hollow is greater than the expansion in solid |
| 2. | the expansion in hollow is the same as that in solid |
| 3. | the expansion in hollow is lesser than in solid |
| 4. | the temperature of both must be the same |
\(\gamma\)-decay occurs (always) when:
| 1. | energy is released due to conversion of a proton into neutron. |
| 2. | energy is released due to conversion of neutron into proton. |
| 3. | energy is released due to de-excitation of nucleus. |
| 4. | none of these. |
Young's moduli of two wires \(A\) and \(B\) are in the ratio \(10:4\). Wire \(A\) is \(2~\text{m}\) long and has radius \(R\). Wire \(B\) is \(1.6~\text{m}\) long and has radius \(2~\text{mm}\). If the two wires stretch by the same length for a given load, then the value of \(R\) is close to:
| 1. | \(\sqrt{2} ~\text{mm} \) | 2. | \(\dfrac {1} {\sqrt{2}}~\text{mm} \) |
| 3. | \(2\sqrt{2} ~\text{mm} \) | 4. | \(2~\text{mm} \) |
A force \(\vec{F}=\hat{i}+2\hat{j}+3\hat{k}~\text{N}\) acts at a point \(\hat{4i}+3\hat{j}-\hat{k}~\text{m}\). Let the magnitude of the torque about the point \(\hat{i}+2\hat{j}+\hat{k}~\text{m}\) be \(\sqrt{x}~\text{N-m}\). The value of \(x\) is:
1. \(145\)
2. \(195\)
3. \(245\)
4. \(295\)
In Young's double slit experiment, the width of one of the slit is three times the other slit. The amplitude of the light coming from a slit is proportional to the slit width. The ratio of the maximum to the minimum intensity in the interference pattern is:
1. \(1:4\)
2. \(3:1\)
3. \(4:1\)
4. \(2:1\)
If the time period of a \(2\) m long simple pendulum is \(2\) s, the acceleration due to gravity at the place where the pendulum is executing simple harmonic motion is:
1. \(\pi^{2}\) m/s2
2. \(2\pi^{2}\) m/s2
3. \(9.8\) m/s2
4. \(16\) m/s2
An electromagnetic wave of frequency \(5~\text{GHz},\) is travelling in a medium whose relative electric permittivity and relative magnetic permeability both are \(2.\) Its velocity in this medium will be:
| 1. | \( 1.5 \times 10^7~\text{m/s}\) | 2. | \( 1.5 \times 10^8 ~\text{m/s}\) |
| 3. | \( 3 \times 10^7~\text{m/s}\) | 4. | \( 3 \times 10^8~\text{m/s}\) |
An airplane, with its wings spread 10 m is flying at a speed of 180 km/h in a horizontal direction. Earth's magnetic field at that point is 4 × 10–4 Wb/m2 and the angle of dip is 30°. The emf induced between the tips of the plane wings will be:
1. 100 mV
2. 150 mV
3. 200 mV
4. 250 mV
Train \(A\) and train \(B\) are running on parallel tracks in opposite directions with speeds of \(36~\text{km/h}\) and \(72~\text{km/h}\), respectively. A person is walking in train \(A\) in the direction opposite to its motion with a speed of \(1.8~\text{km/hr}\). Speed (in m/s) of this person as observed from train \(B\) will be close to:
(Take the distance between the tracks as negligible)
1. \(30.5~\text{ms}^{-1}\)
2. \(29.5~\text{ms}^{-1}\)
3. \(31.5~\text{ms}^{-1}\)
4. \(28.5~\text{ms}^{-1}\)
A body has an initial velocity of \(3~\text{m/s} \) and has an acceleration of \(1~\text{m/s}^2 \) normal to the direction of the initial velocity. Its velocity \(4~\text{s}\) after the start will be:
| 1. | \(7~\text{m/s}\) along the direction of the initial velocity. |
| 2. | \(7~\text{m/s}\) along the normal to the direction of the initial velocity. |
| 3. | \(7~\text{m/s}\) midway between the initial direction and the direction normal to the initial direction. |
| 4. | \(5~\text{m/s}\) at an angle of \({{\tan}}^{-1}(4/3) \) with the direction of the initial velocity. |
The pressure of an ideal gas is written as \(P=\dfrac{2E}{3V}\). Here \(E \) refers to:
| 1. | translational kinetic energy |
| 2. | rotational kinetic energy |
| 3. | vibrational kinetic energy |
| 4. | total kinetic energy |
The echo of a gunshot is heard \(10\) seconds after it is fired. If the velocity of sound is \(330~\text{m/s},\) the distance of the surface which reflects the sound is:
1. \(33~\text{m}\)
2. \(3300~\text{m}\)
3. \(135~\text{m}\)
4. \(1650~\text{m}\)


| 1. | The torque acting on the dipole is zero. |
| 2. | The force acting on the dipole due to the electric field produced by \(Q\) is zero. |
| 3. | The potential energy of the dipole due to the point charge \(Q\) is \( \dfrac{Qp}{4{\mathit{\pi}\varepsilon}_{0}{r}^{2}}.\) |
| 4. | The potential energy of the dipole is maximum. |
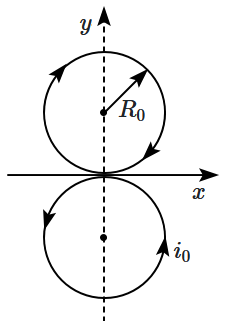
| 1. | \(\left({{i}_{0}{\pi}{R}_{0}^{2}}\right)\sqrt{2} \) | 2. | zero |
| 3. | \({i}_{0}\times{2}{\pi}{R}_{0}^{2} \) | 4. | \({i}_{0}\left({{4}{\pi}{R}_{0}}\right) \) |
| 1. | translate only |
| 2. | rotate only |
| 3. | translate as well as rotate |
| 4. | neither translate nor rotate |


| 1. | \(48\sqrt{2}\) hours | 2. | 48 hours |
| 3. | \(\frac{48}{\sqrt{2}}\) hours | 4. | 24 hours |

| 1. | \(\dfrac{m}{\sin6\mathop {0}\nolimits^{\circ}}\) | 2. | \(\dfrac{m}{\tan6\mathop {0}\nolimits^{\circ}}\) |
| 3. | \(m\sin6\mathop {0}\nolimits^{\circ}\) | 4. | \(\dfrac{m}{\tan3\mathop {0}\nolimits^{\circ}}\) |
| 1. | smaller than objective |
| 2. | small but greater than objective |
| 3. | large |
| 4. | any of the above three |

| 1. | \(1~\text{kg}\) | 2. | \(1.4~\text{kg}\) |
| 3. | \(0.7~\text{kg}\) | 4. | \(2.5~\text{kg}\) |
| 1. | \(3nRT_0\) | 2. | \(9nRT_0\) |
| 3. | \(\dfrac{3}{2}{nRT}_{0}\) | 4. | \(\dfrac{9}{2}{nRT}_{0}\) |
| 1. | \(\dfrac{x}{y}\) | 2. | \(\dfrac{y}{x}\) |
| 3. | \(\dfrac{x}{{x}{-}\left({{z}{-}{y}}\right)}\) | 4. | \(\dfrac{x}{{x}{-}{z}}\) |
| 1. | decreases |
| 2. | increases |
| 3. | remains the same |
| 4. | may increase or decrease depending on the semiconductor |
| 1. | oscillatory circuit | 2. | current amplification |
| 3. | voltage amplification | 4. | all of the above |
| 1. | in accordance with the particle nature of the electron |
| 2. | in accordance with wave nature compatibility of electron |
| 3. | in accordance with Hund's rule |
| 4. | in accordance with Maxwell's theory |
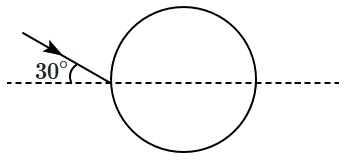
| 1. | \({3}\mathop {0}\nolimits^{\circ}{-}{2}{\sin}^{{-}{1}}\left(\dfrac{1}{2n} \right) \) |
| 2. | zero |
| 3. | \({6}\mathop {0}\nolimits^{\circ}{-}{2}{\sin}^{{-}{1}} \left(\dfrac{1}{2n} \right)\) |
| 4. | \({3}\mathop {0}\nolimits^{\circ}{-}{8}{\sin}^{{-}{1}}\left(\dfrac{1}{n} \right)\) |
| 1. | change in the frequency of light into a change in the electric current. |
| 2. | change in the frequency of light into a change in electric voltage. |
| 3. | change in the intensity of illumination into a change in photoelectric current. |
| 4. | change in the intensity of illumination into a change in the work function of the photocathode. |
| 1. | flow of current |
| 2. | opposite to the flow of current |
| 3. | perpendicular to the flow of current |
| 4. | at an angle of \(45^\circ\) to the flow of current |
A cricket ball is thrown at a speed of \(28\) m/s in a direction \(30^\circ\) above the horizontal. The time taken by the ball to return to the same level is:
| 1. | \(2.5\) s | 2. | \(2.9\) s |
| 3. | \(3.5\) s | 4. | \(3\) s |
Two equal capacitors are first connected in series and then in parallel. The ratio of the equivalent capacitances, \(\left ( \dfrac{C_{Series}}{C_{Parallel}} \right )\) in these two cases will be:
1. \(4:1\)
2. \(2:1\)
3. \(1:4\)
4. \(1:2\)
A cell \(E_1\) of emf \(6~\text{V}\) and internal resistance \(2~\Omega\) is connected with another cell \(E_2\) of emf \(4~\text{V}\) and internal resistance \(8~\Omega\) (as shown in the figure). The potential difference across points \(X\) and \(Y\) is:
 1. \(10~\text{V}\)
1. \(10~\text{V}\)A fluid is flowing through a horizontal pipe of varying cross-sections, with speed \(v\) ms-1 at a point where the pressure is \(P\) pascal. At another point where pressure is \(\dfrac{P}{2}\) pascal, its speed is \(V\) ms-1. If the density of the fluid is \(\rho\) kg-m-3 and the flow is streamlined, then \(V\) is equal to:
| 1. | \(\sqrt{\dfrac{P}{2\rho }+v^{2}}\) | 2. | \(\sqrt{\dfrac{P}{\rho }+v^{2}} \) |
| 3. | \(\sqrt{\dfrac{2P}{\rho }+v^{2}}\) | 4. | \(\sqrt{\dfrac{P}{\rho }+v^{}}\) |
If the potential energy between two molecules is given by \(U = -\dfrac{A}{r^6}+ \dfrac{B}{r^{12}}, \) then the potential energy at equilibrium separation between molecules is:
1. \(\dfrac{-A^{2}}{2B}\)
2. \(\dfrac{-A^{2}}{4B}\)
3. \(0\)
4. \(\dfrac{-A^{2}}{3B}\)
\(n\) moles of a perfect gas undergoes a cyclic process \(ABCA\) (see figure) consisting of the following processes.
| I. | \(A\rightarrow B:\) Isothermal expansion at temperature \(T\) so that the volume is doubled from \(V_1\) to \(V_2=2V_1\) and pressure changes from \(P_1\) to \(P_2\). |
| II. | \(B\rightarrow C:\) Isobaric compression at pressure \(P_2\) to initial volume \(V_1\). |
| III. | \(C\rightarrow A:\) Isochoric change leading to the change of pressure from \(P_2\) to \(P_1.\) |
The work done in the isothermal process is:

1. \(0\)
2. \(nRT\left(\ln2+\frac{1}{2}\right)\)
3. \(nRT\ln2\)
4. \(nRT\left(\ln2-\frac{1}{2}\right)\)
In connection with the circuit drawn below, the value of the current flowing through \(2~\text{k}\Omega\) resistor is:
1. \(20\times10^{-4}~\text{A}\)
2. \(25\times10^{-4}~\text{A}\)
3. \(30\times10^{-4}~\text{A}\)
4. \(35\times10^{-4}~\text{A}\)
A proton, a deuteron, and an \(\alpha\text-\)
| 1. | \(2:2:1\) | 2. | \(4:2:1\) |
| 3. | \(2:1:1\) | 4. | \(1:1:2\) |
A force of \(-F~\hat{k} \) acts on \(O\), the origin of the co-ordinate system. The torque about the point \((1,-1)\) is:

1. \(F(\hat{i} - \hat{j})\)
2. \(-F(\hat{i} - \hat{j})\)
3. \(F(\hat{i} + \hat{j})\)
4. \(-F(\hat{i} + \hat{j})\)
The temperature at which the velocity of sound in air becomes double its velocity at \(0^\circ \text{C}\) is:
| 1. | \(435^\circ \text{C}\) | 2. | \(694^\circ \text{C}\) |
| 3. | \(781^\circ \text{C}\) | 4. | \(819^\circ \text{C}\) |
| 1. | not changed | 2. | halved |
| 3. | doubled | 4. | tripled |

| 1. | \(\sqrt{\dfrac{GM}{d}}\) | 2. | \(\sqrt{\dfrac{2GM}{d}}\) |
| 3. | \(\sqrt{\dfrac{GM}{2d}}\) | 4. | \(\sqrt{\dfrac{4GM}{d}}\) |
| 1. | \(310~\text{eV}\) | 2. | \(620~\text{eV}\) |
| 3. | \(1240~\text{eV}\) | 4. | \(2480~\text{eV}\) |
| 1. | electric field outside the sphere is zero. |
| 2. | electric field inside the sphere is zero. |
| 3. | net induced charge on the sphere is zero. |
| 4. | electric potential inside the sphere is zero. |