Zn and Cd metals do not show variable valency because :
1. They have only two electrons in the outermost subshells
2. Their d-subshells are completely filled
3. Their d-subshells are partially filled
4. They are relatively soft metals
Which of the following metal is expected to have the highest third ionization enthalpy?
1. Cr(Z = 24)
2. V(Z = 23)
3. Mn(Z = 25)
4. Fe(Z = 26)
Aluminium is extracted from alumina Al2O3 by electrolysis of a molten mixture of
1.
2.
3.
4.
Extraction of gold and silver involves leaching with CN- ion. Silver is later recovered by:
1. Distillation
2. Zone refining
3. Displacement with Zn
4. Liquation
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
1. Fe.
2. Zn
3. Mg
4. Cu
The correct match among the following is-
| Column I | Column II |
| A) Cyanide process | 1) Ultra-pure Ge |
| B) Froth floatation Process | 2) Dressing of ZnS |
| C) Electrolytic | 3) Extraction of Al Reduction |
| D) Zone refining | 4) Extracting of Au |
1. A-2, B-3, C-1, D-4
2. A-1, B-2, C-3, D-4
3. A-3, B-4, C-2, D-1
4. A-4, B-2, C-3, D-1
Among the following an iron mineral is:
1. Malachite
2. Cassiterite
3. Pyrolusite
4. Magnetite
A major impurity in pig iron is:
1. Carbon
2. Silicon
3. Phosphorus
4. Manganese
Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
1. Carbon and hydrogen suitable reducing agents for metal sulphides
2. The of the sulphide is greater than those for and
3. the is negative for roasting of sulphur ore to oxide.
4. Roasting of the sulphide to the oxide is thermodynamically feasible
Sulphide ores of metals are usually concentrated by froth flotation process. Which one of the following sulphide ores offers an exception and is concentrated by chemical leaching?
1. Argentite
2. Galena
3. Copper pyrite
4. Sphalerite
Identify the incorrect statement
| 1. | The scientific and technological process used for isolation of the metal from its ore is known as metallurgy |
| 2. | Minerals are naturally occurring chemical substances in the earth's crust |
| 3. | Ores are minerals that may contain a metal |
| 4. | Gangue is an ore contaminated with undesired materials |
In the conversion of Zinc ore to zinc metal, the process of roasting involves
1.
2.
3.
4.
The extraction of silver is achieved by the initial complexation of the ore (Argentine) with X followed by reduction with Y. X and Y respectively are
1. and Zn
2. and Cu
3. and Zn
4. and Zn
Van Arkel method of purification of metals involves converting the metal to a
1. Volatile compound
2. Volatile unstable compound
3. Non-volatile stable compound
4. Non-volatile unstable compound
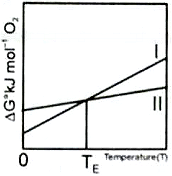
Which of the following is correct?
I. G for reaction (i) is more negative at T < 1125K
II. G for the reduction of CO is more negative at T < 1125K
III. is a better reducing agent at T < 1125K
IV. is a better reducing agent at T > 1125K
1. I and II
2. I and III
3. III only
4. I and IV
Which of the following reactions is an example of auto reduction?
1.
2.
3.
4.
A number of elements are available in earth’s crust but most abundant elements are ____________.
1. Al and Fe
2. Al and Cu
3. Fe and Cu
4. Cu and Ag
Zone refining is based on the principle that ___________.
| 1. | Impurities of low boiling metals can be separated by distillation. |
| 2. | Impurities are more soluble in molten metal than in solid metal. |
| 3. | Different components of a mixture are differently adsorbed on an adsorbent. |
| 4. | Vapors of the volatile compounds can be decomposed in pure metal. |
In the metallurgy of aluminium ________________.
| 1. | Al3+ is oxidized to Al(s). |
| 2. | Graphite anode is oxidized to carbon monoxide and carbon dioxide. |
| 3. | Oxidation state of oxygen changes in the reaction at the anode. |
| 4. | Oxidation state of oxygen changes in the overall reaction involved in the process. |
Electrolytic refining is used to purify which of the following metals?
1. Cu and Zn
2. Ge and Si
3. Zr and Ti
4. Zn and Hg
Answer the question on the basis of Figure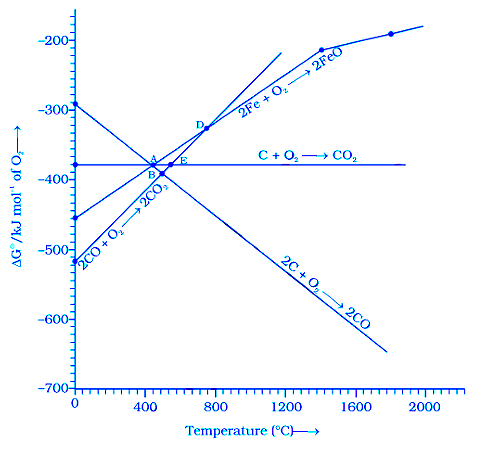
Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
1. Below temperature at point A.
2. Approximately at the temperature corresponding to point A.
3. Above temperature at point A but below the temperature at point D.
4. Above temperature at point A.
Match the items of Column I with items of Column II and assign the correct code:
Column I Column II
(A) Pendulum (1) Chrome steel
(B) Malachite (2) Nickel steel
(C) Calamine (3)
(D) Cryolite (4)
(5)
1. A (1) B (2) C (3) D (4)
2. A (2) B (4) C (5) D (3)
3. A (2) B (3) C (4) D (5)
4. A (4) B (5) C (3) D (2)
Match the items of Column I with the items of Column II and assign the correct
code :
| Column I | Column II | ||
| (A) | Colored bands | (1) | Zone refining |
| (B) | Impure metal to volatile complex | (2) | Fractional distillation |
| (C) | Purification of Ge and Si | (3) | Mond Process |
| (D) | Purification of mercury | (4) | Chromatography |
| (5) | Liquation | ||
1. A =(1); B=(2); C=(4); D=(5)
2. A=(4); B=(3); C=(1); D=(2)
3. A=(3); B=(4); C=(2); D=(1)
4. A=(5); B=(4); C=(3); D=(2)
Match the items of Column I with the items of Column II and assign the correct code :
Column I Column II
(A) Sapphire (1)
(B) Sphalerite (2) NaCN
(C) Depressant (3) Co
(D) Corundum (4) ZnS
(5)
1. A (3) B (4) C (2) D (1)
2. A (5) B (4) C (3) D (2)
3. A (2) B (3) C (4) D (5)
4. A (1) B (2) C (3) D (4)
Match the items of Column I with items of Column II and assign the correct
code :
| Column I | Column II | ||
| (A) | Blistered Cu | (i) | Aluminium |
| (B) | Blast furnace | (ii) | 2Cu2O + Cu2S → 6Cu + SO2 |
| (C) | Reverberatory furnace | (iii) | Iron |
| (D) | Hall-Heroult process | (iv) | FeO + SiO2 → FeSiO3 |
| (V) | 2Cu2S + 3O2 → 2Cu2O + 2SO2 | ||
| Options: | (a) | (b) | (c) | (d) |
| 1. | ii | iii | iv | i |
| 2. | i | ii | iii | v |
| 3. | v | iv | iii | ii |
| 4. | iv | v | iii | ii |
Which of the following is not a mineral of aluminium?
1. Bauxite
2. Cryolite
3. Gibsite
4. Malachite
In the metallurgy of copper, the slag is
1. FeSiO3
2. CaCO3
3. CaSiO3
4. CaO
Galena contains ZnS with PbS. Which of the following is used as depressant to stop ZnS to come with foam?
1. NaCN
2. Aniline
3. Pine Oil
4. H2O
In Ellingham diagrams of oxide formation , which of the following graphs has negative slope?
1. C CO
2. Fe Fe2O3
3. Mg MgO
4. All of the above
Which of the following is leached with NaCN?
1. Ore of Al
2. Ore of Cu
3. Ore of Ag
4. Ore of Zn
Vapour phase refining can be carried out in case of
(1) Ni
(2) Zr
(3) Ti
(4) All of the above
In the metallurgy of copper, matte contains
1. Cu2S + Cu2O
2. Cu2S + FeS
3. FeS + FeO
4. Cu2O + FeO
Which of the following metal is purified by distillation process?
1. Zn
2. Fe
3. Al
4. Cu
The purest form of iron is-
1. Cast iron
2. Steel
3. Wrought iron
4. Grey cast iron
Out of following which metal can be purified by Van Arkel method?
(1) Ti
(2) Zr
(3) Hf
(4) All of the above
Which is mismatched?
1. Zincite - ZnO
2. Calamine -
3. Siderite -
4. Malachite -
The ore having two different metal atoms is
1. Haematite
2. Galena
3. Magnetite
4. Copper pyrites
Which of the following is not a carbonate ore?
1. Siderite
2. Zincite
3. Calamine
4. Dolomite
For which of the following metals, distillation method is used for purification ?
1. Zn
2. Cd
3. Hg
4. All of the above
Carbon cannot be used in the reduction of because
1. The enthalpy of formation of is more than that of
2. Pure carbon is not easily available
3. The enthalpy of formation of is very high
4. It is an expensive process
In the blast furnace at lower temperature, following reaction take place
Compound A and B respectively are
1.
2.
3.
4. FeO and CaO
Which of the following is not an ore of copper?
1. Chalcopyrites
2. Malachite
3. Azurite
4. Epsomite
Which is correct match?
1. Aluminium : Calamine
2. Copper : Malachite
3. Magnesium : Bauxite
4. Zinc : Carnallite
Which of the following is the reaction of calcination?
| 1. | 2Ag + 2HCl + [O] \(\xrightarrow[]{∆}\) 2A gCl + H2O |
| 2. | 2ZnS + 3O2 \(\xrightarrow[]{∆}\) 2ZnO + 2SO2 |
| 3. | MgCO3 \(\xrightarrow[]{∆}\) MgO + CO2 |
| 4. | 2Zn + O2 \(\xrightarrow[]{∆}\) 2ZnO |
In the extraction of copper from its sulphide ore, the metal finally obtained by the reduction of cuprous oxide with
1. Iron (II) sulphide
2. Carbon monoxide
3. Copper (I) sulphide
4. Sulphur dioxide
Roasting of sulphides gives the gas 'X' as a by-product. This is a colourless gas with the choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as an oxidising agent, and its acid has never been isolated. The gas 'X' is:
1. SO2
2. CO2
3. SO3
4. H2S
When is oxidised by in alkaline medium, converts into-
1.
2.
3.
4.
Which one of the following statements related to lanthanum is incorrect?
1. Europium shows +2 oxidation state.
2. The basicity decreases as the ionic radius decreases from pr to Lu.
3. All the lanthanoids are much more reactive than aluminum.
4. Ce+4 solution are widely used as the oxidizing agents in volumetric analysis.
The reason for greater range of oxidation states in actinoids is attributed to:
1. Actinoid Contraction.
2. 5f, 6d and 7s levels having comparable energies.
3. 4f and 5d levels being close in energies.
4. The radioactive nature of actinoids
Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7solution?
1. The solution is decolorized.
2. SO2 is reduced.
3. Green coloured Cr2(SO4)3 is formed.
4. The solution turns blue.
Gadolinium belongs to 4f series. It’s atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
1.
2.
3.
4.
Assuming complete ionization, same moles of which of the following compounds will require the the least amount of acidified KMnO4 for complex oxidation?
1.
2.
3.
4.
Magnetic moment 2.84 BM is given by (At. no. Ni = 28, Ti = 22, Cr = 24, Co = 27)
Identify the alloy containing a non-metal as a constituent in it.
1. Invar
2. Steel
3. Bell metal
4. Bronze
For the four successive transition elements (Cr, Mn, Fe and Co), the correct order of stability of +2 oxidation state among the following is:
(At. No Cr= 24, Mn= 25, Fe=26, Co= 27)
1. Fe>Mn>Co>Cr
2. Co>Mn>Fe>Cr
3. Cr>Mn>Co>Fe
4. Mn>Fe>Cr>Co
The acidified \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) solution turns green when \(\mathrm{Na}_2 \mathrm{SO}_3\) is added to it. This is due to the formation of:
| 1. | \(\mathrm{CrO}_4^{2-} \) | 2. | \(\mathrm{Cr}_2 \mathrm({SO}_3)_3 \) |
| 3. | \(\mathrm{CrSO}_4 \) | 4. | \(\mathrm{Cr}_2 \mathrm({SO}_4)_3\) |
A colored ion in aqueous solutions among the following is:
1. La3+ (Z=57)
2. Ti3+ (Z=22)
3. Lu3+ (Z=71)
4. Sc3+ (Z=21)
The incorrect statement among the following is:
| 1. | There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu. |
| 2. | Lanthanoid contraction is the accumulation of successive shrinkages. |
| 3. | As a result of lanthanoid contraction, the properties of the 4d series of the transition elements have no similarities with the 5d series of elements. |
| 4. | The shielding power of 4f electrons is quite weak. |
When neutral or faintly alkaline is treated with
potassium iodide, the iodide ion is converted into 'X'. The formula of 'X' is:
1.
2.
3.
4.
MnO2 when fused with KOH and oxidized in air gives a dark green compound X. In acidic solution, X undergoes disproportionation to give an intense purple compound Y and MnO2. The compounds X and Y, respectively are
1. K2MnO4 and KMnO4
2. Mn2O7 and KMnO4
3. K2MnO4 and Mn2O7
4. KMnO4 and K2MnO4
Among the following metals, the strongest reducing agent is
1. Ni
2. Cu
3. Zn
4. Fe
How many moles of Br₂ are formed when 2 moles of potassium permanganate react with excess potassium bromide in an aqueous acidic medium?
1. One (1)
2. Three (3)
3. Two (2)
4. Five( 5)
The number of moles of KMnO4 required to oxidize one equivalent of KI in the presence of sulfuric acid is
1. 5
2. 2
3. 1/2
4. 1/5
Lanthanide contraction is caused due to
1. The appreciable shielding on outer electrons by 4f electrons from the nuclear charge
2. The appreciable shielding on outer electrons by 5d electrons from the nuclear charge
3. The same effective nuclear charge from Ce to Lu
4. The imperfect shielding on outer electrons by 4f electrons from the nuclear charge
Europium (Eu) and Terbium (Tb) attain stable 4f configuration by exhibiting oxidation states of:
| 1. | +2 and +4 | 2. | +3 and +4 |
| 3. | +2 and +3 | 4. | +1 and +3 |
Four statements for Cr and Mn are given below:
| (I) | Cr2+ and Mn3+ have the same electronic configuration. |
| (II) | Cr2+ is a reducing agent while Mn3+ is an oxidizing agent. |
| (III) | Cr2+ is an oxidizing agent while Mn3+ is a reducing agent. |
| (IV) | Both Cr and Mn are oxidizing agents. |
The correct statements are
1. (I), (III),(IV)
2. (I) , (II)
3. (I), (II) and (IV)
4. (I), (IV)
Cyanide ion is a very good complexing agent and also functions as a reducing agent. Hence may cyanide complexes of metals are known. Addition of an aqueous solution of KCN to a solution of copper sulphate yields a white precipitate which is soluble in excess of aqueous KCN to form the complex
1. [Cu(CN)4]1-
2. [Cu(CN)4]2-
3. [Cu(CN)4]3-
4. [Cu(CN)4]4-
Electronic configuration of a transition element X in +3 oxidation state is [Ar]3d5 .What is its atomic number?
1. 25
2. 26
3. 27
4. 24
The electronic configuration of Cu(II) is 3d9 whereas that of Cu(I) is 3d10. Which of the following is correct?
1. Cu(II) aq is more stable
2. Cu(II) aq is less stable
3. Cu(I) aq and Cu(II) aq are equally stable
4. Stability of Cu(I) aq and Cu(II) aq depends on nature of copper salts
Generally transition elements form coloured salts due to the presence of unpaired electrons. Which of the following compounds will be coloured in solid state?
1. Ag2SO4
2. CuF2
3. ZnF2
4. Cu2Cl2
The magnetic nature of elements depends on the presence of unpaired electrons. Identify the configuration of transition element, which shows highest magnetic moment.
1. 3d7
2. 3d5
3. 3d8
4. 3d2
The oxidation state common for all lanthanoids is:
1. +2
2. +3
3. +4
4. +5
When KMnO4 solution is added to oxalic acid solution, the decolourisation is slow in the beginning but becomes instantaneous after some time because
1. CO2 is formed as the product.
2. Reaction is exothermic.
3. MnO4– catalyses the reaction.
4. Mn2+ acts as autocatalyst.
There are 14 elements in actinoid series. Which of the following elements does not belong to this series?
1. U
2. Np
3. Tm
4. Fm
Identify the amphoteric oxides from the list provided:
1.
2.
3.
4.
Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not the characteristic property of interstitial compounds?
1. They have high melting points in comparison to pure metals.
2. They are very hard.
3. They retain metallic conductivity.
4. They are chemically very reactive.
The reason for not utilizing HCl to acidify the medium in oxidation reactions involving KMnO4 (in an acidic environment) is:
| 1. | Both HCl and KMnO4 act as oxidizing agents. |
| 2. | KMnO4 oxidizes HCl into Cl2 which is also an oxidizing agent. |
| 3. | KMnO4 is a weaker oxidizing agent than HCl. |
| 4. | KMnO4 acts as a reducing agent in the presence of HCl. |
Which has highest melting point?
1. Cr
2. Fe
3. Cu
4. Mo
Which of the following element does not show a variable oxidation state?
1. Fe
2. Mn
3. Cu
4. Zn
With F highest stable oxidation state of Mn is-
1. + 6
2. + 4
3. + 7
4. + 3
oxidised product of X
X in the above reaction can not be:
1.
2.
3.
4.
In dichromate dianion
1. 4 Cr – O bonds are equivalent
2. 6 Cr – O bonds are equivalent
3. All Cr – O bonds are equivalent
4. All Cr – O bonds are non equivalent
Basic oxide is
1. CrO
2.
3.
4.
The pair having similar magnetic moment is
1.
2.
3.
4.
Reason for lanthanoid contraction is:
1. Negligible screening effect of 'f' orbitals
2. Increasing nuclear charge
3. Decreasing nuclear charge
4. Decreasing screening effect
Which of the statement is not true?
1. On passing through acidified solution, a milky colour is observed
2. is preffered over in volumetric analysis
3. solution in acidic medium is orange
4. solution becomes yellow in increasing the pH beyond 7
| 1. | The greater metallic character of the lanthanoids than that of the corresponding actinoids. |
| 2. | More energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals. |
| 3. | The lesser energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals. |
| 4. | The more active nature of the actinoids. |
The fusion of chromite ore (FeCr2O4) with Na2CO3 in air gives a yellow solution upon the addition of water. Subsequent treatment with H2SO4 produces an orange solution.
The yellow and orange colours, respectively, are due to the formation of:
1. Na2CrO4 and Na2Cr2O7
2. Cr(OH)3 and Na2Cr2O7
3. Cr2(CO3)3 and Fe2(SO4)3
4. Cr(OH)3 and Na2CrO4
Iron does not get oxidised in:
| 1. | Rusting of iron sheets |
| 2. | Decolourisation of blue CuSO4 solution by iron |
| 3. | Formation of Fe(CO)5 from Fe |
| 4. | Liberation of H2 from steam by iron at a high temperature |