What mass of isobutylene is obtained from 37 g of tertiary butyl alcohol by heating with 20% H2SO4 at 363 K, if the yield is 65%?
1. 16 g
2. 18.2 g
3. 20 g
4. 22 g
Which is gem dihalide?
1.
2.
3.
4. None of the above
Which of the following reacts with water
1. CHCl3
2. CCl4
3. CCl3CHO
4. CH2ClCH2Cl
The commonly used dehydrating agent in the preparation of an ester is :
1. P2O5
2. Anhydride CaCl2
3. Anhydride AlCl3
4.Conc. H2SO4
Which represents Riemer-Tiemann reaction?
| (a) |  |
| (b) |  |
| (c) |  |
| (d) |  |
The best reagent to convert pent-3-en-2-ol into pent-3-en-2-one is:
1. Acidic KMnO4
2. Alkaline K2Cr2O7
3. Chromium anhydride in glacial acetic acid
4. Pyridinium Chlorochromate
Dialkyl sulphides are known as:
1. sulphonal
2. mercaptan
3. thioethers
4. thioesters
Diethyl ether absorbs oxygen to form:
1. red colored sweet smelling compound
2. acetic acid
3. ether suboxide
4. ether peroxide
The product A in the below mentioned reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The most acidic compound among the following is:
| 1. | |
2. |  |
| 3. |  |
4. |  |
Ethyl alcohol is denatured by:
1. Methanol and formic acid
2. KCN
3. CH3OH and C6H6
4. CH3OH and pyridine
Consider the following alcohols,
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
Ease of dehydration of the above alcohols will be in the order of:
| 1. | I < II < III < IV | 2. | I > II > III > IV |
| 3. | III < II < I < IV | 4. | II < III < IV < I |
The reaction products of C6H5-O-CH3+ HI
1. C6H5OH + CH3l
2. C6H5l + CH3OH
3. C6H5CH3 +HOI
4. C6H6 + CH3Ol
The number of moles of HI
consumed by the given compound is:
| 1. | 1 | 2. | 2 |
| 3. | 3 | 4. | 4 |
R-CH2-CH2OH can be converted into RCH2CH2COOH. The correct sequence of reagents is:
1. PBr3, KCN, H+
2. PBr3, KCN, H2
3. KCN, H+
4. HCN, PBr3, H+
The reaction of 

1.
2.
3.
4. 
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

The product (x) of this reaction is
1. 
2.
3.
4.
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The major product of the following reaction sequence will be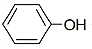
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Compound Y, C7H8O is insoluble in water, dil HCI and aqueous NaHCO3. It dissolves in dilute NaOH. When Y is treated with bromine water it is converted rapidly into a compound of formula C7H5OBr3. The structure of Y is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
What is the correct order of dehydration rate for the compounds (i), (ii), and (iii) when treated with concentrated \(\text{H}_2\text{SO}_4\)?
| (i) |  |
| (ii) |  |
| (iii) |  |
1. (i) > (iii) > (ii)
2. (i) > (ii) > (iii)
3. (ii) > (i) > (iii)
4. (ii) > (iii) > (i)
What sequence of reagents is required to accomplish the following transformation?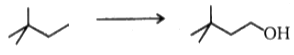
1. (1) NBS, ROOR (2) CH3CH2O- (3)2HBr (4) NH-2 (5) disiamylborane (6) H2O2,OH-
2. (1) Cl2hv (2) OH-, heat; (3) 2HCl (4) OH-,heat (5) HgSO4,H2SO4
3. (1) NBs,ROOR; OH-,DMSO
4. (1) Br2,hv (2) t-butoxide (3) BH3,THF (4) H2O2,OH-
Consider the following compounds:

What is the correct decreasing order of reactivity of given alcohols towards substitution with HBr?
| 1. | III > I > IV > II |
| 2. | III > I > II > Ⅳ |
| 3. | I > III > IV > II |
| 4. | I > III > II > IV |
Predict the product when the given compound reacts with LiAlH4 :

| 1. |  |
2. |  |
| 3. |  |
4. |  |
Identify the product (P) in the given reaction:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The structure of A in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |