When CH3CH2CHCl2 is treated with NaNH2, the product formed is:
1. CH3-CH=CH2
2. CH3-CCH
3. CH3CH2CH
4. CH3CH2C
Which set fo reagents will produce freon ()?
1. C + +
2. +
3. + HF
4. +
Identify (Z) in the following reaction reaction series,
C2H5I (X) (Y) (Z):
1. CH3-CH2-CN
2.
3.
4.
Debromination of meso-dibromobutane gives mainly
1. n-Butane
2. 1-Butene
3. cis-2-Butene
4. trans-2-Butene
Arrange the following compouds in the order of leaving group ability
| (I) |  |
(II) |  |
| (III) |  |
(IV) |  |
1. I > II > III > IV
2. IV > III > I > II
3. III > II > I > IV
4. II > III > IV > I
End product D of the following reaction will be
| (1) |  |
| (2) | |
| (3) |  |
| (4) |  |
Which hydrogen can the Br radical abstract the most easily?
1. a
2. b
3. c
4. d
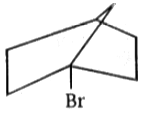
Which of the following reactant will not favour SN2 nucleophilic substitution reaction?
1. 
2. Ph-Br
3.
4. All of the above
What is the relative rate of reaction of the following amine when treated with methyl iodide?
1. A > B > C
2. A > C > B
3. B > C > A
4. B > A > C
Major product of the above reaction is:
1.
2.
3.
4.
Major product of the reaction is:
1.
2.
3.
4.
End product (D) in the given sequence is:
The major product obtained in the reaction of 1-phenyl-2-bromobutane with NaOMe is :
1. trans-1 -phenylbut-1-ene
2. cis-1-phenylbut-2-ene
3. 1-phenyl-2-ethoxybutane
4. cis-1-phenylbut-2-ene
Ph-CH-CH2-CH2 → Product
l l Zn-Cu
Br Br Δ
Product of the above reaction is:-
(1) Ph-CH=CH-CH2-Br
(2) Ph-◁
(3) PH-CHBr-CH=CH2
(4) Ph-C C-CH3
Consider the following reaction,
The product is :
1. a primary amine
2. a tertiary amine
3. a secondary amine
4. a quaternary ammonium salt

Major product (A) is:
1.
2.
3.
4.
Alkyl halides can be obtained by all methods except:
1.
2.
3.
4.
Ether cannot be produced as a major product by the reaction of:
| 1. | CH3CH2Cl + Ag2O(dry) → |
| 2. | (CH3)3CCl + CH3CH2O-Na+ → |
| 3. |  |
| 4. |  |
PCl5 reacts with propanone, to give:
1. gem dichloride
2. vic dichloride
3. propanal
4. propane chloride
Which process does not occur during formation of CHCl3 from C2H5OH and bleaching powder?
1. Hydrolysis
2. Oxidation
3. Elimination
4. Chlorination
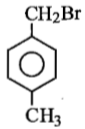
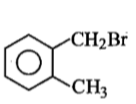
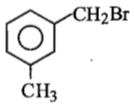
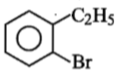
Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
1. 
2. 
3. 
4. 
The halide which undergoes nucleophilic substitution most readily is:
(1) p-H3CC6H4Cl
(2) o-H3COC6H4Cl
(3) p-ClC6H4Cl
(4) p-O2NC6H4Cl