-D-glucose and -D glucose have a specific rotation of +112 and +19 respectively. In aqueous solution the rotation becomes +52. This process is called:
1. inversion
2. racemisation
3. mutarotation
4. embolism
Diastereomers can be separated by:
1. Recrystallization
2. simple distillation
3. electrophoresis
4. all of these
The number of isomers for the aromatic compound of the formula C7H8O is-
1. 2
2. 3
3. 4
4. 5
The structure of diphenylmethane is given below:

The number of structural isomers possible when one of the hydrogen atom is replaced by a chlorine atom are :
| 1. | 6 | 2. | 4 |
| 3. | 8 | 4. | 7 |
Which of the following is more basic than aniline?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
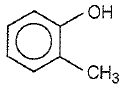
Which one of the following compounds is most acidic?
1. 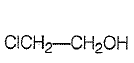
2. 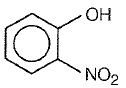
3. 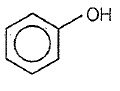
4. 
| 1. | 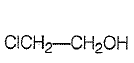 |
| 2. |   |
| 3. |   |
| 4. |   |
Polar solvent favours keto form while non-polar solvent faours enol form. Which has maximum enolic content in CCl4?
1.
2.
3.
4.
What is major product of following reaction ?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
1.
2.
3. 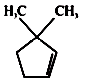
4. 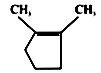
Trans-2-butene on bromination using CS2 gives which dibromoderivative
1. (R), (R)-2,3-dibromobutane
2. (S),(S)-2,3-dibromobutane
3. Racemic-2,3-dibromobutane
4. Meso-2,3-dibromobutane
Which of the following carbocations is expected to be most stable?
1.
2.
3.
4.
In the given conformation C2 is rotated about C2-C3 bond anticlockwise by an angle of 120° then the conformation obtained is
1. Fully eclipsed conformation
2. Partially eclipsed conformation
3. Gauche conformation
4. Staggered conformation
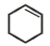
Which of the following is formed by the thermal decomposition of the hydroxide of: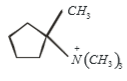
1. 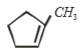
2. 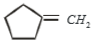
3. 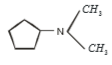
4. 
In the above compound, arrange the nitrogen according to their decreasing basic strength.
1. 1>2>3>4
2. 4>3>1>2
3. 2>4>1>3
4. 3>4>1>2
How many geometrical isomers are possible of the following?
CH3-CH=CH-CH=CH-CH3
1. 2 2. 3
3. 4 4. 6
Most stable radical is
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Correct IUPAC name of the compound
1. 4-(Ethyl methanolyonxy)phenylpropanoate
2. Ethyl 4-propanoyloxybenzenecarboxylate
3. 4-(1-Oxo-2-oxabutyl)phenylpropanoate
4. 1-(1-Oxo-2-oxbutyl)-4-(1-oxopropoxy)benzene
The IUPAC name of the following compound is
1. 2-(Ethoxycarbonyl)benzoylchloride
2. Ethyl 2-(chlorocarbonyl)benzoate
3. Ethyl 2-(chloromethanoyl)benzoate
4. Methyl 2-(Chlorocarbonyl)benzene carboxylate.
A hydrocarbon (R) has six membered ring in which there is no unsaturation. Two alkyl groups are atttached to the ring adjacent to each other. One group has 3 carbon atoms with branching at 1st carbon atom of chain and another has 4 carbon atoms. The larger alkyl group has main chain of three carbon atoms of which second carbon is substituted. Correct IUPAC name of compound (R) is
(1) 1-(1-Methylethyl)-2-(1-methylpropyl)cyclohexane
(2) 1-(2-Methylethyl)-2-(1-methylpropyl)cyclohexane
(3) 1-(1-Methylethyl)-2-(2-methylpropyl)cyclohexane
(4) 1-(1-Methylethyl)-2-butylcyclohexane
In which reaction a chiral reactant is giving a chiral product.
(1) 


The correct stability order of following species is :
(1) x > Y > w > z
(2) y > x > w > z
(3) x > w > z > y
(4) z > x > y > w
Ordinarily the barrier to rotation about a carbon-carbon double bond is quite high but in compound P double bond between two rings was observed by NMR to have a rotational energy barrier of only about 20 cal.\mol., showing that it has lot of single bond charcter. The reason for this is
(1) Double bond having partial triple bond charcter because of resonance
(2) Doule bond undergo flipping
(3) Double bond having very high single bond charcter because of aromaticity gained in both three and five membered rings.
(4) +I effect of nC3H7 groups makes double bond having partial single bond character.
In which of the followig molecules all the effects namely inductive, mesomeric and hyperconjugation operate ?
(1) 


The acid strength order is:
(1) I > IV > II > III
(2) III > I > II > IV
(3) II > III > I > IV
(4) I > III > II > IV
Acid strength of the conjugate acids of the following are-
(1) I > II > III > IV
(2) III > II > I > IV
(3) IV > III > II > I
(4) None of these
If 
1. P
2. Q
3. R
4. S
The correct acidic strength order of acidic hydrogen x, y and z is respectively.
(1) x > z > y
(2) x > y > z
(3) z > y > x
(4) y > z > x
The correct basic strength order is:
(1) I > II > IV > III
(2) IV > III > II > I
(3) III > II > IV > I
(4) III > IV > II > I
(1) R
(2) S
(3) R, S both
(4) None of these
The products of hydrolysis of
(1) HOCH2CH2CH2CH2 CHO + CH3CHO
(2) HOCH2CH2CH2CH2OH + CH3CHO
(3) HOCH2CH2CH2CH2CHO + C2H5OH
(4) HOCH2CH2CH2CH2CH2OH + C2H5OH
The product A is :
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
Which of the following carbocation will undergo rearrangement ?
1. 
2.
Consider the following reaction:-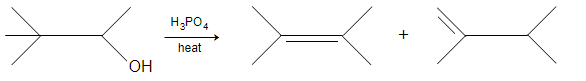
Which response contains all the correct statement about this process?
(1)Dehydration
(2)E2 mechanism
(3)Carbon skeleton migration
(4)Most stable alkene will form
(5)Single-step reaction
(a)1,3 (b)1,2,3 (c)1,2,5 (d)1,3,4
The electrophilic aromatic substitution proceeds through a:
(1) free radical
(2) sigma complex
(3) benzyne
(4) carbene
The IUPAC name of the below given compound is:
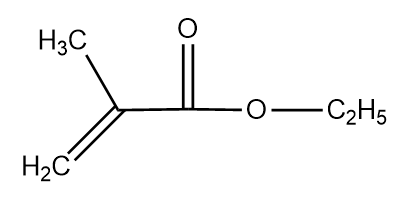
1. Ethyl 2-methylprop-2-enoate
2. Ethyl 2-methylprop-1-enoate
3. 1-Ethoxy 2-methylprop-2-enoate
4. 1-Ethoxy 2-methylprop-2-enal
The IUPAC name of 
1. 2-Phenylpropan-1-ol
2. 2-Phenylpropan-3-ol
3. 1-(2-Hydroxy-1-methylethyl)benzene
4. 1-((Hydroxymethyl)ethyl)benzene
The IUPAC name of 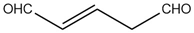 is:
is:
(1) propene-1, 3-dial
(2) Propene-1, 3-dicarbaldehyde
(3) Pent-3-ene-1, 5-dial
(4) Pent-2-ene-1, 5-dial
The IUPAC name of
is:
(1) Butane-1,4-dicarbonitrile
(2) Ethane-1, 2-dicarbonitrile
(3) Ethane-1, 2-dinitrile
(4) Butane-1, 4-dinitrile
The IUPAC name of 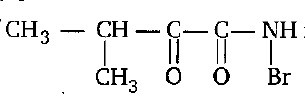 is:
is:
1. (N-Bromo)-2-keto-3-methylbutanamide
2. (Bromo) -2-keto-4-methylbutanamide
3. (N-Bromo)-1, 2-diketo-3-methylbutanamine carboxamide
4. (N-Bromo)-1-keto-2-methylpropane
The IUPAC name of the below-given compound is:
1. 2-(Bromomethyl)-3-oxopentane carboxamide
2. 1-Bromo-2-carbamoylpentan-3-one
3. 5-Bromo-4-carbamoylpentan-3-one
4. 2-(Bromomethyl)-3-oxopentanamide
The IUPAC name of 
1. 2-Phenyl ethanone
2. 1-Phenyl ethanone
3. 1-(Oxoethyl)benzene
4. 1-(Ethyloxo)-benzene
The IUPAC name of the following compound is:
(1) 4-Bromo-3-cyanophenol
(2) 2-Bromo-5-hydroxybenzonitrile
(3) 2-cyano-4-hydroxybromobenzene
(4) 6-Bromo-3-hydroxybenzonitrile
The species that contains only three pairs of electrons among the following is-
| 1. | Carbocation | 2. | Carbanion |
| 3. | Free radical | 4. | None of the above |
The sigma bond energy of C-H bond in C2H6 is:
(1) 99 kcal
(2) 140 kcal
(3) 200 kcal
(4) 60 kcal
Which of the following is strongest nucleophile?
1. Br-
2.
3.
4.
(CH3)4N+ is neither an electrophile, nor a nucleophile because it:
1. does not have electron pair for donation as well as cannot attract electron pair
2. neither has electron pair available for donation nor can accommodate electron since all shells of N are fully occupied.
3. can act as Lewis acid and base
4. none of the above
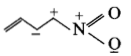
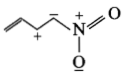
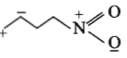
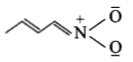
The least stable resonance structure among the following is:
1. 
2. 
3. 
4. 
Hyperconjugation involves the overlap of the following orbitals:
1. σ - σ
2. σ - p
3. p - p
4. π - π
The correct stability order for the following species is:
(1) (II) > (IV) > (I) > (III)
(2) (I) >(II) > (III) >(IV)
(3) (II) > (I) > (IV) > (III)
(4) (1) > (III) > (II) > (IV)
In E2 elimination, some compounds follow Hofmann's rule which means:
1. the double bond goes to the most substituted carbon
2. the compound is resistant to elimination
3. no double bond is formed
4. the double bond goes mainly towards the least substituted carbon
SN1 reaction on optically active substrates mainly gives:
1. Retention in configuration
2. Inversion in configuration
3. Racemic product
4. No product is obtained
Amongst the following which of the above are true for SN2 reaction?
(i) The rate of reaction is independent of the concentration of the nucleophile
(ii) The nucleophile attacks the carbon atom on the side of the molecule opposite to the group being displaced.
(iii) The reaction proceeds with simultaneous bond formation and bond rupture.
1. (i), (ii)
2. (i), (iii)
3. (i), (ii), (iii)
4. (ii), (iii)
Carbanions initiate:
(1) addition reactions
(2) substitution reaction
(3) both (1) and (2)
(4) none of these
Ease of abstraction of hydrogen is greater when attached to:
(1) 1 carbon
(2) 2 carbon
(3) 3carbon
(4) neo carbon
Carbanion can undergo:
1. rearrangement
2. combination with cation
3. addition to a carbonyl group
4. all of the above are correct
The SN1 mechanism for substitution reaction by nucleophile is favored by:
1. Low concentration of nucleophile
2. Weak nature of the nucleophile
3. Polar solvent
4. All of the above
The SN2 mechanism for R-X + KOH (aq) R-OH + KX follows with:
(1) 100% inversion
(2) 50% inversion
(3) 40% inversion
(4) 30% inversion
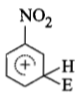
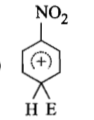
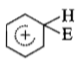
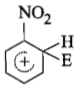
The electrophile. E attacks the benzene ring to generate the intermediate - complex. Of the following which - complex is of lowest energy?
1. 
2. 
3. 
4. 
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S, the rates of the reaction vary as: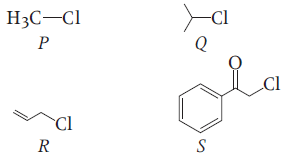
1. P > Q > R > S
2. S > P > R > Q
3. P > R > Q > S
4. R > P > S > Q
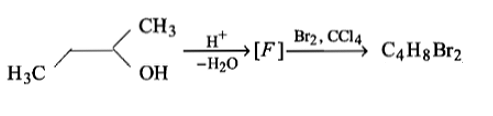
How many structures of F are possible?
(1) 2
(2) 5
(3) 6
(4) 3
In pyrrole,
The electron density is maximum on
1. 2 and 3 2. 3 and 4
3. 2 and 4 4. 2 and 5
The correct order of strengths of the carboxylic acids :-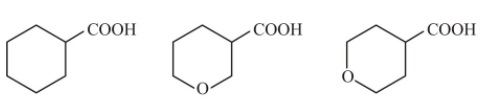
(a) I>II>III
(b) II>III>I
(c) III>II>I
(d) lI>I>IlI
Which of the following meta-directing substituents in aromatic substitution is the most deactivating?
1. -C≡N
2. -SO3 H
3. -COOH
4. -NO2
CH3-CHCl-CH2-CH3 has a chiral centre. Which one of the following represents it's R configuration ?
The number of isomeric structures for C2H7N would be:
1. 4
2. 3
3. 2
4. 1
Which of the following is the most stable carbocation
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In the following compounds,
The order of basicity is
1. IV>I>III>II
2. III>I>IV>II
3. II>I>III>IV
4. I>III>II>IV
Correct IUPAC name of the compound 
(1) 2-Ethyl-3-methylbut-2-ene-1,4-dioic anhydride
(2) 3-Ethyl_2-methylbut-2-enedioic anhydride
(3) 2-Ethyl-3-Methyl-1,4-diketobut-2-enoic anhydride
(4) 2-Ethyl-3-methylcyclopenatanoxy-1,4-dione
The IUPAC name of the compound is:
1. 2-Methyl-6-oxohex-3-enamide
2. 6-Keto-2-methyl hexanamide
3. 2-Carbamoylhexanal
4. 2-Carbamoylex-3-enal
The IUPAC name of the given compound is:
1. 2-Phenylpropan-3-al
2. Formylethylbenzene
3. 2-Phenylpropanal
4. Ethylformylbenzene
The IUPAC name of the given compound is:
1. Tris(chloromethyl)methane
2. 1,3-Dichloro-2-(chloromethyl)propane
3. 1-Chlorobis(chloromethyl) ethane
4. None of the above
The IUPAC name of 
(1) 3-Carboxy-3-hydroxypentanedicarboxylic acid
(2) 2-Hydroxypropane-1, 2, 3-tricarboxylic acid
(3) 2-Hydroxypropane-1, 2, 3-trioic acid
(4) 3-Hydroxypropane-1, 2, 3-tricarboxylic acid
The IUPAC name of 
(1) N, N-Dimethyl aminobenzene
(2) N, N-Dimethyl benzenamine
(3) (1) and (2) both are correct
(4) none of these
Which of the following is singlet carbene?
1. (CH3)3C+
2. C2H5-CH
3.
4. CH2 =CH-
The number of isomers for the compound with molecular formula C2BrCIFI is:
(1) 3
(2) 4
(3) 5
(4) 6
In Duma's method of estimation of nitrogen, 0.35 g of an organic compound gave 55 ml of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be:
(Aqueous tension at 300 K = 15 mm)
1. 16.45
2. 27.45
3. 44.45
4. 35.45
How many stereoisomers does this molecule have?
CH3CH=CHCH2C HBrCH3
1. 4
2. 6
3. 8
4. 2