A solid has a structure in which w atoms are located at the corners of a cubic lattice. Oxygen atom at the center of the edges and Na atom at the center of the cube. The formula of the compound is :
1. NaWO2
2. NaWO3
3. Na2WO3
4. NaWO4
An element (atomic mass = 100 g mole) having BCC structure has a unit cell edge of 400 pm. The density of the element is (number of atom bcc(z)=2)
1. 2.144 g/cm
2. 5.2 g/cm
3. 7.289 g/cm
4. 10.376 g/cm
The radius of Ag+ ion is 126 pm and that I- ion is 216 pm. The coordination number of Ag+ ion is
1. 2
2. 4
3. 6
4. 8
The analysis shows that an oxide ore of nickel has formulaNi0.98O1.00 The percentage of nickel as Ni3+ ions are nearly
1. 2
2. 96
3. 4
4. 98
The relationship between osmotic pressure at 273K, when 10 gm glucose (P1), 10 gm urea (P2), and 10 gm sucrose (P3) dissolved in 250 ml of water is
1. P1>P2>P3
2. P3>P1>P2
3. P2>P1>P3
4. P2>P3>P1
If 'A' contains 2% NaCl and is separated by a semi-permeable membrane from 'B', which contains 10% NaCl, which event will occur?
1. NaCl will flow from 'A' to 'B'
2. NaCl will flow from 'B' to 'A'
3. Water will flow from 'A' to 'B'
4. Water will flow from 'B' to 'A'
The azeotropic mixture of water and ethanol boils at a temperature lower than that of either pure water or pure ethanol. This indicates that the mixture exhibits:
1. No deviation from Raoult's law
2. Positive deviation from Raoult's law
3. Negative deviation from Raoult's law
4. The solution is unsaturated
What is the required amount of K₂SO₄ in moles to dissolve in 12 moles of water to decrease
the vapour pressure by 10 mmHg, if the vapour pressure of pure water is 50 mmHg?
1. 3 mol
2. 0.5 mol
3. 1 mol
4. 2 mol
Which has the highest freezing point?
1. 0.01 M NaCl
2. 0.05M Urea
3. 0.01 M MgCl2
4. 0.02 M NaCl
When mercuric iodide is added to the aqueous solution of potassium iodide is
1. Freezing point is raised
2. Freezing point is lowered
3. Freezing point does not change
4. The boiling point does not change
In the following reaction: XA YB where the -ve sign indicates the rate of disappearance of the reactant. Then X: Y is
1. 1:2
2. 2:1
3. 3:1
4. 3:10
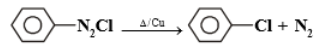
Half-life is independent of the concentration of the reactant. After 10 minutes volume of N2 gas is 10L and after complete reaction, it is 50 L. Hence the rate constant is
1. log 5 min-1
2. log 1.25 min-1
3. log 2 min-1
4. log 4 min-1
The activation energies of the forward and backward reactions in the case of a chemical reaction are 30.5 and 45.4 KJ /mol respectively. The reaction is
1. Exothermic.
2. Endothermic.
3. Neither exothermic nor endothermic.
4. Independent of temperature.
If NaCl is doped with 10-4 mol % of SrCI, the concentration of cation vacancies will be (NA = 6.02 x 1023 mol-)
1. 6.02 x 1016 mol-1
2. 6.02 x 1017 mol-1
3. 6.02 x 1014 mol-1
4. 6.02 x 1015 mol-1
AB crystallizers in a body-centred cubic lattice with edge length a equal to 387 pm. The distance between two oppositely charged ions in the lattice is
1. 250
2. 200
3. 300
4. 335
The Van't Haff factor of a 0.005 M aqueous solution of KCl is 1.95. The degree of ionization of KCl is:
1. 0.95
2. 0.97
3. 0.94
4. 0.96
By how much will the potential of half cell Cu2+|Cu change if the solution is diluted to 100 times at 298 K?
1. Increases by 59 mv
2. Decreases by 59 mv
3. Increases by 29.5 mv
4. Decreases by 29.5 mv
The half-life period of a first-order reaction is 10 min. The time required for the concentration of the reactant to change from 0.08 M to 0.02 M is:
1. 10 min
2. 20 min
3. 30 min
4. 40 min