The property of water that enables it to
stabilize temperature in bodies of organisms
and thus makes it indispensible for life is:
1. High specific heat
2. High latent heat of fusion
3. High latent heat of vaporization
4. High cohesion and adhesion
The element that makes up the highest
percent weight of the human body is:
1. Carbon
2. Hydrogen
3. Oxygen
4. Nitrogen
The number of high energy bonds in a molecule
of ATP is:
1. 1
2. 2
3. 3
4. 4
The molecule that is never branched and is
straight is:
1. Amylose
2. Amylopectin
3. Glycogen
4. Cellulose
An amino acid that is negative charged amongst
the following will be:
1. Glutamic acid
2. Lysine
3. Arginine
4. Histidine
The correct ascending order of percent
composition of various components of a typical
cell would be:
1. Protein – Carbohydrate – Lipids – Nucleic acids
2. Protein – Nucleic acids – Carbohydrate – Lipids
3. Carbohydrates – Protein – Nucleic acids– Lipids
4. Carbohydrates – Protein – Lipids – Nucleic
acids
Type of bonds that stabilize a tertiary structure
of a protein include all the following except:
1. van der Waal’s interactions
2. Hydrophobic interactions
3. Disulfide linkage
4. Covalent bonds
Protein molecules that assist in the proper
folding of other proteins are known as:
1. Ubiquitins
2. Chaperonins
3. Prions
4. Calmodulins
A ribosome inhibiting secondary metabolite
protein found in the castor plant is:
1. Ricin
2. Abrin
3. Concanvalin A
4. Vinblastin
When an enzyme-substrate reaction tends
toward zero order, the only way to make a
reaction speed up is to:
1. Add more substrate
2. Add more enzyme
3. Increase the temperature
4. Increase the pressure in the medium
The Glucose transpoter protein present in the
baso-lateral surface of the intestinal epithelial
cells is:
1. GLUT 1
2. GLUT 2
3. GLUT 3
4. GLUT 4
The plot represents the relationship between
substrate concentration and velocity for a
single enzyme in the absence (curve x) and
presence (curve y) of a compound that binds
allosterically to the enzyme. The allosteric
compound is: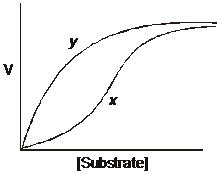
1. a competitive inhibitor.
2. a noncompetitive inhibitor.
3. an irreversible inhibitor.
4. an activator.
The metal ion that acts as a cofactor for both
alcohol dehydrogenase and carbonic anhydrase
is:
1. Magnesium
2. Iron
3. Zinc
4. Nickel
The living state can be best described as a:
1. non equilibrium steady state
2. non equilibrium non steady state
3. equilibrium non steady state
4. equilibrium steady state
The blood concentration of glucose ina normal
healthy individual is:
1. 3.5 – 4.0 mM
2. 4.0 – 4.5 mM
3. 4.5 – 5.0 mM
4. 5.0 – 5.5 mM
All the following assumptions apply to
Michaelis-Menten kinetic analyses of enzyme
action EXCEPT
1. the total enzyme concentration studied at each
substrate concentration is fixed in analysis of
enzyme kinetics.
2. formation of enzyme-substrate complex does
not appreciably decrease the concentration of
substrate.
3. Km decreases with competitive inhibition.
4. maximal velocity is reached when the enzymesubstrate complex is equal to the total concentration of enzyme present.
The conversion of glucose into lactic acid in
our muscles takes place in:
1. 9 metabolic steps
2. 10 metabolic steps
3. 11 metabolic steps
4. 12 metabolic steps
The living state is synonymous with:
1. Cellular organization
2. Metabolism
3. Consciousness
4. Reproduction
In case of incomplete dominance, the F1
progeny of a monohybrid cross resemble:
1. Either of the parents
2. The male parent
3. Neither of the parents
4. Both parents
What is applicable to both Klinefelter’s
syndrome and Turner’s syndrome?
I. They result from non-disjunction of sex
chromosomes during gamete formation
II. They can be identified by a karyotype
III. They have 44 autosomes
1. I and II only
2. I and III only
3. II and III only
4. I, II and III
An exchange of segments between nonhomologous chromosomes is called as:
1. Crossing over
2. Inversion
3. Reciprocal translocation
4. Transposing
Suppose two genes A and B are located on the
same chromosome but are 60 map units apart.
What fraction of the progeny from the cross
AB/ab x ab/ab would be Ab/ab?
1. 60%
2. 50%
3. 30%
4. 25%
If a father and son are both affected by redgreen colour blindness, then what can be said
definitely?
I. The son has received the trait from the father.
II. The mother of the affected son has to be
affected by red-green colour blindness.
III. Any sister of the affected son can never be
affected by red-green colour blindness,
1. I and II only
2. I and III only
3. II and III only
4. None
If individuals of genotype AaBbCc are
intercrossed, how many different phenotypes
can appear in their offspring?
1. 3
2. 6
3. 8
4. 9
Genes A and B are farther apart than are genes
A and C, and all three are linked. What cannot
be concluded?
1. B might be between A and C.
2. C might be between A and B.
3. A might be between B and C.
4. More crossovers will occur between A and B
than between A and C.
A man of which of the following blood groups
could not be the father of a group O child?
1. A
2. B
3. O
4. AB
Two garden pea dihybrids with round seed
shape and yellow seed color [RrYy] are
intercrossed. Assuming independent
assortment, the gene for seed shape is
inherited by the progeny in a phenotypic ratio
of:
1. 3 : 1
2. 1 : 1
3. 9 : 3 : 3 : 1
4. 1 : 1 : 1 : 1
The gene for the enzyme Phenylalanine
hydroxylase is located on chromosome:
1. 7
2. 11
3. 12
4. X
A human geneticist determined the pedigree
shown in the diagram with filled symbols
showing the affected individuals. How is this
pattern of inheritance described?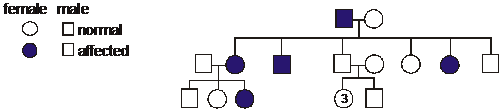
1. autosomal dominant
2. autosomal recessive
3. sex-linked recessive
4. sex-linked dominant
Consider the following statements regarding
Y chromosome in humans:
I. Y chromosome is sex-determining chromosome
in humans
II. Y chromosome contains a gene, SRY, which
triggers embryonic development as a male
III. 50 % of sons of a male inherit his Y
chromosome
Of the given statements, the correct
statements are:
1. I and II only
2. I and III only
3. II and III only
4. I, II and III
Mating between black rats of identical
genotype produced offspring as follows: 14
cream colored, 47 black and 19 albino. This
ratio can be explained if the pheneomenon
exhibited here is:
1. Recessive epistasis
2. Polygenic inheritance
3. Multiple allelism
4. Incomplete penetrance
Identify the incorrect statement regarding test
cross?
1. It is used to determine the genotype of an
individual exhibiting a dominant phenotype.
2. The testcross parent is always homozygous
recessive for all of the genes under
consideration.
3. The purpose of the testcross is to discover how
many different kinds of gametes are being
produced by the individual whose genotype is
in question.
4. A homozygous dominant individual will produce
two types of progeny and a heterozygous
individual will produce only one type of progeny
Sickle cell disease is an example of:
1. Sex linked recessive disorder
2. Pleiotropy
3. Polygenic trait
4. Sex influenced trai
Unattached earlobes is dominant to attached
earlobes. Two parents, both with unattached
earlobes, had a child with attached earlobes.
What are the chances that their next child will
have attached earlobes?
1. 0 %
2. 25 %
3. 50 %
4. 100 %
What decides the frequency of an allele in a
population?
1. Dominance
2. Its location on a particular chromosome
3. Its degree of expression
4. Natural selection
What is the number of linkage groups in
humans?
1. 22
2. 23
3. 24
4. 46
Assuming complete dominance –
recessiveness and independent assortment,
the ratio of progeny with the genotypes AaBb,
AABb, Aabb and aaBb from a cross AaBb X
AABb would respectively be:
1. 4 : 4 : 2 : 0
2. 4 : 2 : 4 : 2
3. 4 : 4 : 2 : 2
4. 4 : 4: 2 : 1
A certain gene has 5 alleles. What will be the
number of possible genotypic combinations?
1. 5
2. 15
3. 32
4. 2
How many unique gametes could be produced
through independent assortment by an
individual with the genotype AaBbCCDdEE?
1. 4
2. 8
3. 16
4. 32
Three babies were mixed up in a hospital. After
consideration of the data below, which of the
following represent the correct baby and parent
combinations?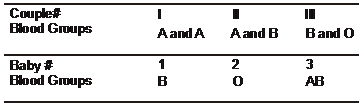
1. I-3, II-1, III-2
2. I-1, II-3, III-2
3. I-2, II-3, III-1
4. I-2, II-1, III-3
Chromosomes and genes share all of the
following characteristics except that
1. they are both present in pairs in all diploid cells.
2. they both undergo segregation during meiosis.
3. their copy numbers in the cell decrease after
meiosis, and increase during fertilization.
4. they both pair up with their homologues during
prophase of mitosis
The improvement of microscopy techniques
in the late 1800s set the stage for the
emergence of modern genetics because
1. it revealed new and unanticipated features of
Mendel’s pea plant varieties.
2. it allowed biologists to study meiosis and mitosis,
revealing the parallels between the behaviors
of genes and chromosomes.
3. it allowed scientists to see the DNA present
within chromosomes.
4. it led to the discovery of mitochondria
When Thomas Hunt Morgan crossed his
red-eyed F1 generation flies to each other, the
F2 generation included both red- and
white-eyed flies. Remarkably, all the
white-eyed flies were male. What was the
explanation for this result?
1. The involved gene was on the X chromosome.
2. The involved gene was on the Y chromosome.
3. The involved gene was on an autosome.
4. Other male-specific factors influence eye color
in flies
Males are more often affected by sex-linked
traits than females because
1. males are hemizygous for the X chromosome.
2. male hormones such as testosterone often
exacerbate the effects of mutations on the X
chromosome.
3. female hormones such as estrogen often
compensate for the effects of mutations on the
X.
4. X chromosomes in males generally have more
mutations than X chromosomes in females
It was important that Mendel examined not
just the F1 generation in his breeding
experiments, but the F2 generation as well,
because:
1. He obtained very few F1 progeny, making
statistical analysis difficult.
2. Parental traits that were not observed in the F1
reappeared in the F2, suggesting that the traits
did not truly disappear in the F1.
3. Analysis of the F1 progeny would have allowed
him to discover the law of segregation, but not
the law of independent assortment.
4. The dominant phenotypes were visible in the
F2 generation, but not in the F1.
Two true-breeding stocks of pea plants are
crossed. One parent has red, axial flowers and
the other has white, terminal flowers; all F1
individuals have red, axial flowers. If 1,000 F2
offspring resulted from the cross,
approximately how many of them would you
expect to have red, terminal flowers? (Assume
independent assortment).
1. 65
2. 190
3. 250
4. 565
An abnormal human of the karyotype 49,
XXXYY will form how many Barr bodies?
1. 1
2. 2
3. 3
4. 4
When the two genes in a dihybrid cross are
situated on the same chromosome:
1. The proportion of parental gene combinations
was much higher than the non-parental type.
2. The proportion of parental gene combinations
was much lesser than the non-parental type.
3. The proportion of parental gene combinations
was equal to the non-parental type.
4. Only recombinants are formed.
Match each item in COLUMN I with one in
COLUMN II and select your answer from the
codes given:
COLUMN I COLUMN II
SCIENTIST CONTRIBUTION
A. Francis Crick a. Breaking the
genetic gode
B. Nirenberg b. Established
Coenorhabditis
elegans as a model
genetics study
organism
C. Benzer c. Central dogma of
molecular biology
D. Brenner d. Bacteriophage
genetics
Codes:
A B C D
1. c a d b
2. c a b d
3. a b c d
4. a c d b
The mechanism of sex determination in
grasshoppers is:
1. XX – XY; male heterogamety
2. XX – XY; female heterogamety
3. XX – XO; male heterogamety
4. XX – XO; female heterogamety
Male pattern baldness is a ___________ trait.
1. Sex-linked
2. Sex-limited
3. Sex-influenced
4. Y-linked
Griffith co-injected the heat killed S and live
R strains of Pneumococcus bacterium into the
mice and much to his surprise the mice
developed pneumonia and died. He concluded
that:
I. Live R were transformed into Live S strain as
he was able to isolate Live S strain from the
blood of the infected mice.
II. Bacterial transformation is a stable and heritable
change as the culture of bacteria isolated from
dead mice were unable to infect other mice.
1. Both I and II are correct
2. Only I is correct
3. Only II is correct
4. Both I and II are incorrect
The radius of the double helix of B-DNA
[Watson and Crick model] is approximately:
1. 1.0 nm
2. 2.0 nm
3. 0.34 nm
4. 3.4 nm
An RNA primer is essential for DNA synthesis
carried out by DNA polymerase III. This is
because, to synthesize DNA, DNA polymerase
III requires:
1. A free 3’ – PO4 group
2. A free 5’ – PO4 group
3. A free 3’ – OH group
4. A free 5’ – OH group
On examining a sample of human DNA, it was
found that it contains approximately 24 %
thymine. Which of the following would be true
based on this finding?
1. The amount of adenine in a similar sample of
DNA from any human being will be
approximately 24 %.
2. The amount of adenine in a similar sample of
DNA from any living being will be approximately
24 %.
3. The amount of adenine in a similar sample of
DNA from any eukaryotic cell will be
approximately 24 %.
4. The amount of adenine in a similar sample of
DNA from any mammal will be approximately
24 %.
Which of the following possible modes of
replication was eliminated by Meselson and
Stahl based on the finding after one generation
of replication of the bacterium?
1. Dispersive
2. Semi-conservative
3. Conservative
4. Both 1 and 3
What would be the effect of a mutation in the
operator region of the lac operon that leads to
inability of the repressor to bind to the
operator?
1. The lac ZYA genes would be inducible by lactose
2. The lac ZYA genes will be repressed by lactose
3. The lac ZYA genes will not be expressed
4. The lac ZYA genes will be expressed constitutively
. It is possible to insert the DNA from one virus
(virus A) into the protein coat of a different
virus (virus B). If such a composite virus
infected a cell, the resultant viruses produced
in the host cell would have DNA like virus
____ and protein like ______.
1. A;B
2. A;A
3. B;B
4. B;A
The mRNA codon GUG codes for:
1. Valine
2. Glutamic acid
3. Tryptophan
4. Formylated methionine
What defines the template and coding strand
of DNA for transcription?
1. Presence of the structural gene in a transcription unit
2. The core RNA polymerase enzyme
3. Presence of the promoter in a transcription unit
4. Presence of 3’ – 5’ TAC codon on the DNA template
Which radioactive isotope was used in Meselson
and Stahl experiment to prove the semiconservative mode of DNA replication?
1. 35S
2. 32P
3. 15N
4. None
In eukaryotes RNA polymerase III
transcribes:
1. hnRNA, snRNAs and 5.8S RNA
2. tRNA, 5.8S RNA and snRNAs
3. tRNA, 5SrRNA and snRNAs
4. rRNAs – 28S, 18S and 5S
Identify the incorrect statement:
1. The DNA of the bacteriophage X 174 has
5386 nucleotides
2. Bacteriophage lambda DNA has 48502 base
pairs
3. The DNA of Escherechia coli has 4.6 X 103
base pairs
4. Haploid content of human DNA is 3.3 X 109
base pairs
In a DNA strand, two nucleotides are linked
through a ___________ linkage to form a
dinucleotide.
1. 3’ – 5’ phoshodiester bond
2. 5’ – 3’ phosphodiester bond
3. 1’ N-glycosidic bond
4. Two or three hydrogen bonds
A mammalian cell typically has 1.2 meters
(when completely outstretched) of double
stranded genomic DNA. The total time to
duplicate the DNA is 5 hours. How many
origins of replication are there if the rate of
duplication is 16µmeters/min?
1. 250 2. 15000
3. 100 4. 500
The bacterial promotor sequence usually found
at the -10 position (ten base pairs upstream of
the transcription start site) is:
1. TTGACA
2. TAGACA
3. TATAAT
4. TATTAT
Under conditions where methionine must be
the first amino acid, what protein would be
coded for by the following mRNA?
5'-CCUCAUAUGCGCCAUUAUAAGUGACACACA-3'
1. pro his met arg his tyr lys cys his thr
2. met arg his tyr lys cys his thr
3. met arg his tyr lys
4. met pro his met arg his tyr lys cys his thr
Replication in prokaryotes differs from
replication in eukaryotes for which of the
following reasons?
1. Prokaryotic chromosomes have histones,
whereas eukaryotic chromosomes do not.
2. Prokaryotic chromosomes have a single origin
of replication, whereas eukaryotic
chromosomes have many.
3. The rate of elongation during DNA replication
is slower in prokaryotes than in eukaryotes.
4. Prokaryotes produce Okazaki fragments during
DNA replication, but eukaryotes do not.
A molecule of tRNA with anticodon AAA will
transport the amino acid:
1. Lysine
2. Methionine
3. Arginine
4. Phenylalanine
A nonsense mutation typically involves
1. Confusion of which amino acids go where in
the polypeptide chain.
2. Expansions of the ends of chromosomes due to
repeated nucleotides.
3. Inappropriate termination of translation early in
the “reading frame”.
4. A mistake that causes a portion of a
chromosome to “flip” in orientation.
Regarding gene expression, which of these
accurately distinguish prokaryotes from
eukaryotes?
1. Nucleotides in the promoter are different in
prokaryotes and eukaryotes.
2. Genes are more widely-spaced apart in
eukaryotes than prokaryotes.
3. Prokaryotic ribosomes look different than
eukaryotic ones.
4. Prokaryotic ribosomes translate mRNA
transcripts that are still being synthesized by
RNA polymerase.
The region of a gene which “tells” RNA
polymerase where the genetic message is
located is called the:
1. Start codon.
2. Promoter.
3. Transcription factor.
4. Initiator.
Which is responsible for the production of a
peptide bond between adjacent amino acids
during translation?
1. Peptidyl transferase activity of the large subunit.
2. Proteins in the large and small subunit of the
ribosome.
3. Ribozyme activity of tRNA.
4. The charging effect of aminoacyl tRNA
synthetase.
Which of the following is likely if there is a
mutation in the lacY gene of the lac operon in
E.coli?
1. The lac genes would be expressed efficiently
only in the absence of lactose.
2. The lac genes would be expressed efficiently
until the lactose supply in the cell is exhausted.
3. The lac genes would be expressed continuously.
4. Expression of the lac genes would cease
immediately.
Consider the following two statements:
I. During transcription, the template strand is read
in a 3'-to-5' direction.
II. During transcription, an RNA is transcribed in
the 5'-to-3' direction.
The correct statements is/are:
1. I only
2. II. Only
3. I and II
4. None
Identify the correct statements from the given
statements and choose the correct option:
I. Both prokaryotic and eukaryotic mRNAs have
a 5' cap.
II. Only prokaryotic mRNAs are polyadenylated
at the 3' end.
III. In prokaryotes, transcription is coupled to
translation.
IV. In eukaryotes, RNA splicing occurs after the
mRNA is transported into the cytoplasm.
V. RNA splicing requires the formation of a
spliceosome.
VI. Both prokaryotic and eukaryotic mRNAs are
synthesized by RNA polymerase.
1. III, V and VI only
2. II, III, V and VI only
3. I, III, V and VI only
4. I, III, IV, V and VI only
A molecule that can act as a genetic material
must fulfill all the following criteria except:
1. It should be able to generate its replica.
2. It should chemically and structurally be stable.
3. It should be able to express itself in the form of
Medelian characters.
4. It should provide scope for rapid changes
required for evolution.
Histones are rich in:
1. Basic amino acids lysine and arginine
2. Acidic amino acids lysine and arginine
3. Basic amino acids glutamate and aspartate
4. Acidic amino acids glutamate and aspartate
The difference between ATP and the
nucleoside triphosphates used during DNA
synthesis is that
1. The nucleoside triphosphates have the sugar
deoxyribose; ATP has the sugar ribose.
2. The nucleoside triphosphates have two
phosphate groups; ATP has three phosphate
groups.
3. ATP contains three high-energy bonds; the
nucleoside triphosphates have two.
4. ATP is found only in human cells; the nucleoside
triphosphates are found in all animal and plant
cells.
In E. coli, there is a mutation in a gene called
dnaB that alters the helicase that normally acts
at the origin. Which of the following would you
expect as a result of this mutation?
1. No proofreading will occur.
2. No replication fork will be formed.
3. The DNA will supercoil.
4. Replication will occur via RNA polymerase
alone.
If pre-mRNA is hybridized with mature mRNA,
regions loop out and can be viewed with an
electron microscope. What do the looped out
regions represent?
1. Excised exons.
2. Introns to be excised later.
3. Exons in the pre-mRNA.
4. Exons that are in the process of being fused
together.
The first genetic code of life was most likely
based on:
1. RNA
2. Single stranded DNA
3. Double stranded DNA
4. Proteins
The lac repressor protein binds to the operator
region...
1. in the absence of lactose
2. in the presence of lactose
3. in the presence of cAMP
4. in the presence of glucose
tRNA molecules are linked to their respective
amino acids by enzymes called:
1. Phenylalanine hydroxylases
2. Beta-galactosidases
3. Ornithine decarboxylases
4. Aminoacyl-tRNA synthetases
Which of the following pairs of codons encode
the same amino acid?
1. AUG and AUC
2. UAA and UAC
3. GUA and GUG
4. UAG and UAC
Which of the following most accurately restates
Mendel’s law of segregation?
1. Genes are inherited in discrete units from one
generation to the next.
2. Genes can exist in different forms, known as
alleles.
3. Homologous chromosomes separate during
gamete formation.
4. Genes on different chromosomes are inherited
independently.
What key characteristic of T2 bacteriophage
allowed Hershey and Chase to use it in their
studies of the genetic material?
1. Its genes encode proteins that assemble to
produce the viral coat.
2. It injects its genetic material into a bacterial cell.
3. It can undergo either the lytic or lysogenic life
cycle.
4. It enters the bacterial cell to cause infection.
Which of the following is a description of
chromatin?
1. All the genetic sequences contained by
members of a particular species.
2. The DNA-protein complex which comprises
eukaryotic chromosomes.
3. Repetitive sequences contained within the
genome of an organism.
4. The protein coding sequences and their
regulatory elements.
What is a key difference between DNA pol III
and DNA ligase?
1. Only DNA pol III synthesizes phosphoester bonds.
2. Only DNA ligase synthesizes phosphoester bonds.
3. DNA pol III can synthesize DNA from 3'-5'.
4. DNA ligase can use energy from ATP rather
than nucleotides.
A recessive pair in garden pea plant will be
1. Round pea shape
2. Yellow seed colour
3. Yello pods
4. Axial flowers
A mixture of carbon monoxide and carbon dioxide is found to have a density of 1.7 g/L at S.T.P. The mole fraction of carbon monoxide is-
1. 0.37
2. 0.40
3. 0.30
4. 0.50
At constant temperature, if pressure increases by 1%, the percentage decrease of volume is-
1. 1%
2. 100/101%
3. 1/101%
4. 1/100%
A flask containing air (open to atmosphere) is heated from 300 K to 500 K. The percentage of air escaped to the atmosphere is nearly-
1. 16.6
2. 40
3. 66
4. 20
The correct order of solubility in an aqueous medium is:
1. CuS > ZnS > Na2S
2. ZnS > Na2S > CuS
3. Na2S > CuS > ZnS
4. Na2S > ZnS > CuS
25.3 g of Sodium carbonate Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ion, Na+ and carbonate ion are respectively (Molar mass of Na2CO3 = 106 g mol-1)
1. 0.955 M and 1.910 M
2. 1.910 M and 0.955 M
3. 1.90 M and 1.910 M
4. 0.477 M and 0.477 M
The correct order of decreasing second ionisation enthalpy of Ti (22), V (23), Cr (24) and Mn (25) is
(1) Cr > Mn > V > Ti
(2) V > Mn > Cr > Ti
(3) Mn > Cr > Ti > V
(4) Ti > V > Cr > Mn
The correct order of increasing thermal stability of K2CO3, MgCO3, CaCO3 and BeCO3 is:
(1) BeCO3 < MgCO3 < K2CO3 < CaCO3
(2) BeCO3 < MgCO3 < CaCO3 < K2CO3
(3) MgCO3 < BeCO3 < CaCO3 < K2CO3
(4) K2CO3 < MgCO3 < CaCO3 < BeCO3
Concentrated aqueous sulphuric acid is 98% by mass and has a density of 1.80 g mL-1. Volume of acid required to make one litre of 0.1 M H2S04 solution is :
(1) 11.10 mL
(2) 16.65 mL
(3) 22.20 mL
(4) 5.55 mL
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2 D). This is because:
(1) in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same direction
(2) in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directions
(3) in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
(4) in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directions
Which of the following statements is correct regarding the element Unununium?
1. It is an inner transition element.
2. It belongs to the 8th period in the periodic table.
3. It is a transition element.
4. It is a non-transition element.
The radius of which ion is closest to that of ion ?
1.
2.
3.
4.
The correct order of is:
(1) Ne>F>O>N
(2) O>F>Ne>N
(3) Ne>O>F>N
(4) O>Ne>F>N
Which of the following compound is most acidic?
1. 2.
3. 4.
If P and T are second period p-block elements then which of the following graph show correct relation between valence electrons in to (corresponding molecules) and their bond order is :
(a) 
(b)
(c) 
(d)
Consider the following changes:
The second ionization energy of M could be calculated from the energy values associated with :
(a) 1+3+4 (b) 2-1+3
(c) 1+5 (d) 5-3
Which of the following statements is incorrect?
| 1. | Van der Waals' radius of iodine is more than its covalent radius. |
| 2. | All isoelectronic ions belong to the same period of the periodic table. |
| 3. | of the nitrogen atom is higher than that of the oxygen atom, while of the oxygen atom is higher than that of the nitrogen atom. |
| 4. | The electron affinity of the nitrogen atom is almost zero while that of the phosphorus atom is 74.3 kJ |
First three ionisation energies (in kJ/mol) of three representative elements are given below:
Element
P 495.8 4562 6910
Q 737.7 1451 7733
R 577.5 1817 2745
Then incorrect option is :
1. Q: Alkaline earth metal.
2. P: Alkali metal.
3. R: s-block element.
4. All three: P,Q & R belong to the same period.
The incorrect statement is:
(1) The second ionisation energy of Se is greater than that of second ionisation energy of As
(2) The first ionisation energy of ion is greater than that of first ionisation energy of ion
(3) The third ionisation energy of F is greater than that of third ionisation energy of O
(4) Helogens have highest I.E. in respective period
The process requiring the absorption of energy is :
1.
2.
3.
4.
Given below are four orders for the size of the species. Choose the correct ones:
(a)
(b)
(c)
(d)
1. (a), (b) & (c)
2. (b), (c) & (d)
3. (a), (c)
4. (a), (b), (c) & (d)
The van der waals' constants for a gas are : . lts Boyle temperature is roughly
(1)
(2)
(3)
(4)
(g) decomposes according to the equation
A sealed container contains 0.5 mol of gas at 100°C and 2 atm pressure. What would be the pressure in the container if the gas is decomposed completely according to the above equation and the temperature were maintained at 100°C –
(A) 0.5 atm
(B) 1.0 atm
(C) 2.0 atm
(D) 3.0 atm
Two flasks A and B of equal volume containing and HCl gases, are connected by a narrow tube of negligible volume. The two gases were prevented from mixing by stopper fitted in connecting tube. For further detail of experiment refer to the given figure. What will be final pressure in each flask when passage connecting two tubes are opened. Assume ideal gas behaviour of and gas and the reaction.
(1) 40 mm Hg
(2) 60 mm Hg
(3) 20 mm Hg
(4) 10 mm Hg
The valves X and Y are opened simultaneously. The white fumes of will first form at :
(1) A
(2) B
(3) C
(4) A, B and C simultaneously
Two flasks of equal volume are connected by a narrow tube (of negligible volume) at 27ºC and contain 0.70 mole of at 0.5 atm. One of the flasks is then immersed in a hot bath, kept at 127 ºC, while the other remains at 27 ºC. The final pressure is -
1. 5.714 atm
2. 0.5714 atm
3. 2.5214 atm
4. 5.5114 atm
The number of electrons present in 9.5 g of \(PO^{3-}_4\) are:
1. \(6\)
2. \(5~ N_A\)
3. \(0.1~ N_A\)
4. \(4.7 ~ N_A\)
Equivalent weight of when it disproportionate into and is:
1. M
2. M/2
3. M/4
4. 3M/4
The density of dry air containing only and is 1.15 g/L at 740 mm and 300 K. What is the percentage
composition of by weight in the air?
| 1. | 78.00% | 2. | 75.50% |
| 3. | 70.02% | 4. | 72.75% |
In preparation of iron from haematite by the reaction with carbon:
How much 80% pure iron could be produced from 120 kg of 90% pure ?
1. 94.5 kg
2. 60.48 kg
3. 116.66 kg
4. 120 kg
A mixture of contain 30.40% mass per cent of nitrogen. What is the mass ratio of the two components in the mixture?
1. 2 : 1
2. 1 : 2
3. 3 : 4
4. 4 : 1
0.607 g of a silver salt of tribasic organic acid was quantitatively reduced to 0.37 g of pure Ag. What is the mol. wt. of the acid?
1. 207 g
2. 210 g
3. 531 g
4. 324g
2.0 g sample contain mixture of and , on very strong heating leave a residue weighing 1.96 g. The reaction responsible for loss of weight is (unbalance equation)
What is the percentage by mass of in original sample?
1. 10%
2. 20%
3. 40%
4. 60%
0.8 mole of a mixture of CO and CO2 requires exactly 40 gram of NaOH in a solution for complete conversion of all the CO2 into Na2CO3. How many moles more of NaOH would it require for conversion into Na2CO3, if mixture (0.8 mole) is completely oxidised to CO2?
1. 0.2
2. 0.6
3. 1
4. 1.5
25.4 g of and 14.2 g of are made to react completely to yield a mixture of ICI and ICI3. The mole of ICI and ICI3 formed, is respectively -
1. 0.5, 0.2
2. 0.1, 0.1
3. 0.1, 0.3
4. 0.3, 0.4
60 mL of a mixture of nitrous oxide and nitric oxide was exploded with excess of hydrogen. If 38 mL of was formed, calculate the volume of each gas in the mixture. All measurements are made at constant P and T.
1.20 ml +30 ml
2. 44 ml + 16 ml
3. 10 ml+40 ml
4 .25 ml +25 ml
The angular momentum of an electron in d orbital is equal to
1.
2.
3.
4. 0 h
The haemoglobin from the red blood corpuscles contains approximately 0.33% iron
by mass. The molar mass of haemoglobin is 67,200. The number of iron atoms in each molecule of haemoglobin is :
(atomic mass of iron=56):
| 1. | 2 |
| 2. | 3 |
| 3. | 4 |
| 4. | 5 |
Which mixture is lighter than humid air?
1.
2.
3.
4.
Which is not correct curve for gay-lusacc's law?
1 .
2.
3.
4. 
Four particles have speed 2, 3, 4 and 5 cm/s respectively. Their rms speed is:
1.
2.
3.
4.
The compressibility factor for nitrogen at 330 K and 800 atm is 1.90 and at 570 K and 200 atm is 1.10. A certain mass of occupies a volume of at 330 K and 800 atm. Calculate volume occupied by same quantity of gas at 570 K and 200 atm:
1. 1 L
2. 2 L
3. 3 L
4. 4 L
The van der Waals parameters for gases W, X, Y and Z are
|
Gas |
a (atm L2 mol-2) |
b(L mol-1) |
|
W |
4.0 |
0.027 |
|
X |
8.0 |
0.030 |
|
Y |
6.0 |
0.032 |
|
Z |
12.0 |
0.027 |
Which one of these gases has the highest critical temperature?
1. W
2. X
3. Y
4. Z
The temperature at which the second virial coefficient of real gas is zero is called:
1. Critical temperature
2. Triple point
3. Boiling point
4. Boyle's temperature
What will happen to gas if it is adiabatically expanded at 600 K if its Boyle's temperature is 290 K?
1. Heating
2. Cooling
3. Constant
4. None
The van der Waal's equation of law of corresponding states for 1 mole of gas is:
1.
2.
3.
4.
Energy due to position of a particle is given by, , where and are constants, y is distance. The dimensions of ( × ) are
1.
2.
3.
4.
A physical quantity x depends on qualities y and z as follows: x = Ay + B tanCz , where A, B and C are constants. Which of the following do not have the same dimensions?
1. x and B
2. C and
3. y and B/A
4. x and A
A dust particle oscillates in air with a time period which depends on atmospheric pressure P, density of air d and energy of the particle E, then time period is proportional to
1.
2.
3.
4.
Which of the following group have different dimension?
1. Potential difference, EMF, voltage
2. Pressure, stress, Young’s modulus
3. Heat, energy, work-done
4. Dipole moment, electric-flux, electric field
If a copper wire is stretched to make its radius decrease by \(0.1\%\), then the percentage increase in resistance is approximately:
1. \(0.1\%\)
2. \(0.2\%\)
3. \(0.4\%\)
4. \(0.8\%\)
Two resistances of 400 and 800 connected in series with a 6 volt battery of negligible internal resistance. A voltmeter of resistance 10,000 is used to measure the potential difference across 400 . The error in the measurement of potential
difference in volts approximately is
1. 0.01
2. 0.02
3. 0.08
4. 0.05
If there is a positive error of 50% in the measurement of velocity of a body, then the error in the measurement of kinetic energy is
1. 25 %
2. 50 %
3. 100 %
4. 125
The diameter of a wire is measured with a screw gauge having \(50\) divisions on circular scale and by one complete rotation of circular scale, main scale moves \(0.5\) mm. If reading of screw gauge is \(0.250\) cm. The minimum percentage error in the reading will be:
1. \(0.4\)
2. \(0.8\)
3. \(4\)
4. \(5\)
The pitch of a screw gauge is 1 mm and there are 100 divisions on its circular scale. When nothing is put in between its jaws, the zero of the circular scale lies 4 divisions below the reference line. When a steel wire is placed between the jaws, two main scale divisions are clearly visible and 67 divisions on the circular scale are observed. The diameter of the wire is:
1. 2.71 mm
2. 2.67 mm
3. 2.63 mm
4. 2.65 mm
What are the dimensions of electrical permittivity?
1.
2.
3.
4.
We have error in the measurement of length, radius, mass and current of a wire are 2%, 3%, 2% and 1% then error in its density will be
1. 11%
2. 8%
3. 10%
4. 7%
The velocity of water waves may depend on their wavelength , the density of water and the acceleration due to gravity g. The method of dimensions gives the relation between these quantities as
1.
2.
3.
4.
A particle located at \(x = 0\) at time \(t = 0,\) starts moving along the positive \(x\text-\)direction with a velocity ‘\(v\)’ that varies as \(v=\alpha\sqrt{x}.\) The displacement of the particle varies with time as:
1. \(t^3\)
2. \(t^2\)
3. \(t\)
4. \(t^{1/2}\)
Position-time curve of a body moving along a
straight line is shown in figure. The velocity-time
curve for the motion of the particle will be
1.
2.
3.
4.
An object is moving with a uniform acceleration which is parallel to its instantaneous direction of motion. The displacement(s) – velocity (v) graph of this object is
1.
2.
3.
4.
A body is thrown up in a lift with an upward velocity u relative to the lift from its floor and the time of flight is found to be t. The acceleration of the lift will be
1.
2.
3.
4.
In a car race, car A takes t0 time less to finish than car B and passes the finishing point with a velocity v0 more than car B. The cars start from rest and travel with constant accelerations a1 and a2. Then the ratio is equal to
1.
2.
3.
4.
Acceleration of a particle moving along a straight line is a function of velocity as . At t = 2s, its velocity v = 16 . Its velocity at t = 3s will be
1. 20
2. 25
3. 30
4. 22.5
A particle moving with uniform retardation along a straight line covers distances a and b in successive intervals p and q seconds. The acceleration of the particle is
1.
2.
3.
4.
A bus is beginning to move with an acceleration of 1 m/s2. A boy who is 48 m behind the bus starts running with constant speed of 10 m/s. The earliest time when the boy can catch the bus is
1. 8 sec
2. 10 sec
3. 12 sec
4. 14 sec
A particle has an initial velocity 11 m/s due east and a constant acceleration of 2 m/s2 due west. The distance covered by the particle in sixth second is
1. zero
2. 0.5 m
3. 1 m
4. 2 m
A point moves in a straight line so that its displacement x metre at time t sec is given by . Its acceleration in m/s2 at time t sec is
1.
2.
3.
4.
A body starts from rest with uniform acceleration and remains in motion for n seconds. If its final velocity after n second is v, then its displacement in the last two seconds will be
1.
2.
3.
4.
Two particles A and B are separated from each other by a distance l. At time t = 0, particle A starts moving with uniform acceleration a along a line perpendicular to the initial line joining A and B. At the same moment, particle B starts moving with acceleration of constant magnitude b (> a) such that particle B always points towards the instantaneous position of A. The distance travelled by B till the moment B converges with A will be
1.
2.
3.
4.
An aeroplane is rising vertically with acceleration f. Two stones are dropped from it at an interval of time t. The distance between them at time t' after the second stone is dropped will be
1.
2.
3.
4.
The moment of the force, at (2, 0, -3), about point (2, -2, -2), is given by
1.
2.
3.
4.
A particle moves so that its position vector is given by . Where is a constant. Which of the following is true?
1. Velocity and acceleration both are perpendicular to
2. Velocity and acceleration both are parallel to
3. Velocity is perpendicular to and acceleration is directed towards the origin
4. Velocity is perpendicular to and acceleration is directed away from the origin.
The position vector of a particle as a function of time is given by :
. Where R is in meter, t is in seconds and denote unit vectors along x- and y-directions, respectively ?
Which one of the following statements is wrong for the motion of particle ?
1. Magnitude of acceleration vector is , where v is the velocity of particle
2. Magnitude of the velocity of particle is 8 meter/second
3. Path of the particle is a circle of radius 4 meter.
4. Acceleration vector is along
If vector \(\overrightarrow{A} = \cos \omega t \hat{i} + \sin \omega t \hat{j}\) and \(\overrightarrow{B} =\cos \frac{\omega t}{2} \hat{i} + \sin \frac{\omega t}{2} \hat{j}\) are functions of time, then the value of \(t\) at which they are orthogonal to each other will be:
1. \(t = \frac{\pi}{2\omega}\)
2. \(t = \frac{\pi}{\omega}\)
3. \(t=0\)
4. \(t = \frac{\pi}{4\omega}\)
Two forces are acting as shown in figure. The resultant of the two forces is
1.
2.
3.
4. None of these
A ship A is moving Westwards with a speed of 10 and a ship B 100 km South of A, is moving Northwards with a speed of 10 . The time after which the distance between them becomes shortest is :
1. 5 h
2.
3.
4. 0 h
A boat is moving with velocity 2i + 3j with respect to ground. The water in the river is moving with a velocity -2j -3j with respect to ground. The relative velocity of the boat with repect to water is
1. 4 j
2. -4 + 6j
3. 4 i + 6j
4. 6 j
The equation of a projectile is y =
The angle projection is given by
1.
2.
3.
4. zero
A particle is projected with a velocity v such that its range on the horizontal plane is twice the greatest height attained by it. The range of the projectile is (where g is acceleration due to gravity)
1.
2.
3.
4.
A projectile can have the same range R for two angles of projection. It and be the times of flight in the two cases, then what is the product of two times of flight?
1.
2.
3.
4.
Two particles start simultaneously from the same point and move along two straight lines, one with uniform velocity v and other with a acceleration a. If is the angle between the lines of motion of two particles then the least value relative velocity will be at a time given by
1.
2.
3.
4.
A particle is projected over a traingle from one end of a horizontal base and grazing the vertex falls on thr other end of the base. If and be the base angles and the angle of projection, then the correct relation between and is
1.
2.
3.
4.
AB is an inclined plane of inclination with horizontal. Point O (point of projection ) is 20 m above point A. A particle is projected horozontally and it collides with the plane AB, perpendicularly.
Speed of the particle must be (g = 10 )
1.
2.
3.
4.
A cricket ball thrown across a field is at height and from point of projectoin at times and respectively after the throw. The ball is caught by a fielder at the same height as that of projection. The time of flight of the ball in this journey is
1.
2.
3.
4. None
If the equation for the displacement of a particle moving on a circular path is given by () = , where is in radian and t in second, then the angular velocity of the particle after 2 s from its start is :
1. 8 rad/s
2. 12 rad/s
3. 24 rad/s
4. 36 rad/s
The values of a, for which points A, B, C with position vectors and respectively are the vertices of a right angled traingle with angle, C = are
1. 2 and 1
2. -2 and -1
3. -2 and 1
4. 2 and -1
A point P moves in counter-clockwise direction on a circular path as shown in the figure. The movement of 'P' is such that it sweeps out a length s = + 5, where s is in meters and t is in seconds. The radius of the path is 20 m. The acceleration of 'P' when t = 2 s is nearly.
1. 13
2. 12
3. 7.2
4. 14
A particle is projected from a tower as shown in figure, then the distance from the foot of the tower where it will strike the ground will be
1. 4000/3m
2. 2000/3m
3. 1000/3m
4. 2500/3m
A projectile is fired with a velocity v at the rigt angle to the slope which is inclined at an angle with the horizontal. The range of the projectile along the inclined plane is :
1.
2.
3.
4.
If rain falls vertically with a velocity wrt wind and wind blows with a velocity from east to west, then a person standing on the roadside should hold the umbrella in the direction
1.
2.
3.
4.