An organic compound (A) contains 69.77% carbon, 11.63% hydrogen, and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic and propanoic acid. Compound A would be:
1. Pentan-2-one
2. Butanone
3. 3-Methylbutanone
4. Propan-2-ol
Subtopic: Isomers & Reaction Mechanism |
66%
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
The below structure is an example of :
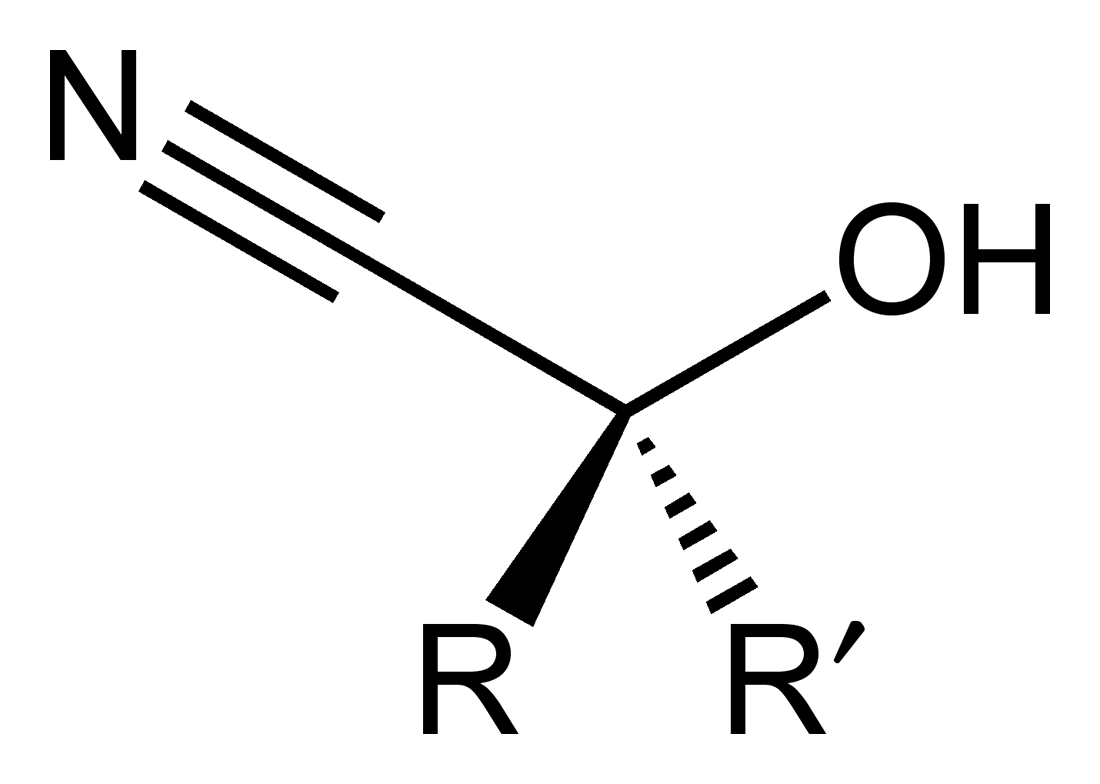
| 1. | Cyanohydrin | 2. | Hemiacetal |
| 3. | Acetal | 4. | Cyanoalcohol |
Subtopic: Isomers & Reaction Mechanism |
70%
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
The major product formed when cyclohexanecarbaldehyde reacts with PhMgBr and H3O+ is:
| 1. |  |
2. |  |
| 3. |  |
4. | None of these |
Subtopic: Aldehydes & Ketones: Preparation & Properties | Isomers & Reaction Mechanism |
84%
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
