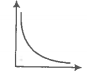
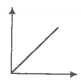
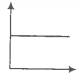
Which of the following statement(s) are correct:
(1) A plot of log KP versus 1/T is linear
(2) A plot of log (X) versus time is linear for a first-order reaction '
(3) A plot of log P versus 1/T is linear at constant volume
(4) A plot of P versus 1/V is linear at a constant temperature.
(1) 1, 2
(2) 2, 4
(3) 2, 3
(4) 1, 4
A plot of volume versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in the figure given below.
The correct order of pressure is -
Temperature (K)
1.
2.
3.
4.
The plots of different values of pressure versus temperature are shown in the given figure. The correct order of volume for the following plot is-
1. V4 < V3 < V2 < V1
2. V4 > V3 < V2 > V1
3. V4 > V3 > V2 > V1
4. V4 > V3 > V2 < V1
The variation of pressure with the volume of the gas at different temperatures can be
graphically represented as per the following graph:
If the temperature increases from 200K to 400K (at constant pressure), the volume of a
gas would-
1. Increase
2. Decrease
3. Remain constant
4. 1st decreases then increases
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
A B C
(1) 2 3 1
(2) 1 2 3
(3) 3 2 1
(4) 1 3 1
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 1 |
Match the following gas law with the equation representing them.
|
A. Boyle’s law |
1. at constant T and p |
|
B. Charle’s law |
2. …constant T,V |
|
C. Dalton’s law |
3. constant |
|
D. Avogadro’s law |
4. at constant n and p |
|
5. at constant n and T |
Codes
| Options: | A | B | C | D |
| 1. | 5 | 4 | 2 | 1 |
| 2. | 3 | 4 | 2 | 1 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
Match the graphs between the following variables with their names.
|
Graphs |
Names |
|
A. Pressure vs temperature graph at constant molar volume |
1. Isotherms |
|
B. Pressure vs |
2. Constant temperature Curve |
|
C. Volume vs temperature graph at constant pressure. |
3. Isochores |
|
4. Isobars |
Codes
| Options: | A | B | C |
| 1. | 3 | 1 | 4 |
| 2. | 1 | 2 | 3 |
| 3. | 5 | 4 | 3 |
| 4. | 4 | 5 | 3 |