Assertion(A): The compressibility factor 'Z' is equal to the ratio of the molar volume of a real gas and the molar volume of an ideal gas.
Reason(R): 'Z' depends upon the pressure of real gas.
1. Both A and R are true and R is the correct explanation of A.
2. Both A and R are true but R is not the correct explanation of A.
3. A is true and R is false.
4. A and R both are false.
Reason(R): At critical temperature gas always shows non-ideal behaviour.
1. Both A and R are true and R is the correct explanation of A.
2. Both A and R are true but R is not the correct explanation of A.
3. A is true and R is false.
4. A and R both are false.
Reason(R): Even at low pressures, repulsive forces dominate in hydrogen gas.
1. Both A and R are true and R is the correct explanation of A.
2. Both A and R are true but R is not the correct explanation of A.
3. A is true and R is false.
4. A and R both are false.
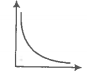
A plot of pressure versus for a gas at constant temperature is given below:
The relation between is-
The variation of pressure with the volume of the gas at different temperatures can be
graphically represented as per the following graph:
If the temperature increases from 200K to 400K (at constant pressure), the volume of a
gas would-
1. Increase
2. Decrease
3. Remain constant
4. 1st decreases then increases
The variation of vapour pressure of different liquids with temperature is shown below:
Boiling points of liquids A and B are, respectively
1. 311 K and 374 K
2. 298 K and 353 K
3. 307 K and 337 K
4. Cannot be determined from the graph given above
Consider the following graph:
The curve that represents CO2 gas is -
1. X
2. Y
3. Both X and Y
4. Cannot predict from the graph
Pressure versus volume graph for a real gas and an ideal gas is shown below:
The correct statement among the following is -
1. Real gases show a large deviation from ideal behaviour at low pressure.
2. Real gases show a very small deviation from ideal behaviour at low pressure.
3. Real gases do not behave ideally under any conditions at low pressure.
4. None of the above statements is correct
The plots of different values of pressure versus temperature are shown in the given figure. The correct order of volume for the following plot is-
1. V4 < V3 < V2 < V1
2. V4 > V3 < V2 > V1
3. V4 > V3 > V2 > V1
4. V4 > V3 > V2 < V1
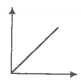
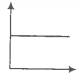
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
A B C
(1) 2 3 1
(2) 1 2 3
(3) 3 2 1
(4) 1 3 1