An ideal gas expands isothermally from at 300 K against a constant pressure of . The work done by the gas is:
1.
+270 kJ
2.
-900 J
3.
+900 kJ
4.
-900 kJ
Reversible expansion of an ideal gas under isothermal and adiabatic conditions are as shown in the figure:
ABIsothermal expansion
ACAdiabatic expansion
Which of the following options is not correct?
1.
2.
3.
4.
A piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant temperature of 37.0ºC. As it does so, it absorbs 208 J of heat. The values of q and w for the process will be-
(R = 8.314 J/mol K) (ln 7.5 = 2.01)
| 1. | q = +208 J, w = -208 J | 2. | q = -208 J, w = -208 J |
| 3. | q = -208 J, w = + 208 J | 4. | q = +208 J, w = + 208 J |
Which is an extensive property?
1. Temperature
2. Chemical potential
3. Gibbs free energy
4. Molar volume
2.1 g of Fe combines with S evolving 3.77 kJ. The heat of formation of FeS in kJ/mole is–
| 1. | – 3.77 | 2. | – 1.79 |
| 3. | – 100.5 | 4. | None of the above |
The pair of isochoric among the transformation of state is:
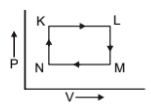
1. K to L and L to M
2. L to M and N to K
3. L to M and M to N
4. M to N and N to K
A process among the following shows decrease in entropy is :
1. )
2. Evaporation of water
3. Expansion of a gas at a constant temperature
4. Sublimation of solid to gas
Under the isothermal condition, a gas at 300 K expands from 0.1 L to 0.25 L against a constant external pressure of 2 bar. The work done by the gas is:
[Given that 1 L bar = 100 J]
1. 30 J
2. -30 J
3. 5 kJ
4. 25 J
Assuming each reaction is carried out in an open container,
Reaction that shows ΔH=ΔE is :
1.
2.
3.
4.
| 1. | Carbon and hydrogen act as suitable reducing agents for metal sulphides |
| 2. | The \(\Delta_f G^0\) of the sulphide is greater than those for \(C S_2\) and \(\mathrm{H}_2 \mathrm{~S}\) |
| 3. | The \(\Delta_f G^0\) is negative for roasting of sulphur ore to oxide. |
| 4. | Roasting of the sulphide to the oxide is thermodynamically feasible |

